Figure 7. Covalent histone modifications modulate Cathepsin L activity and its downstream effects.
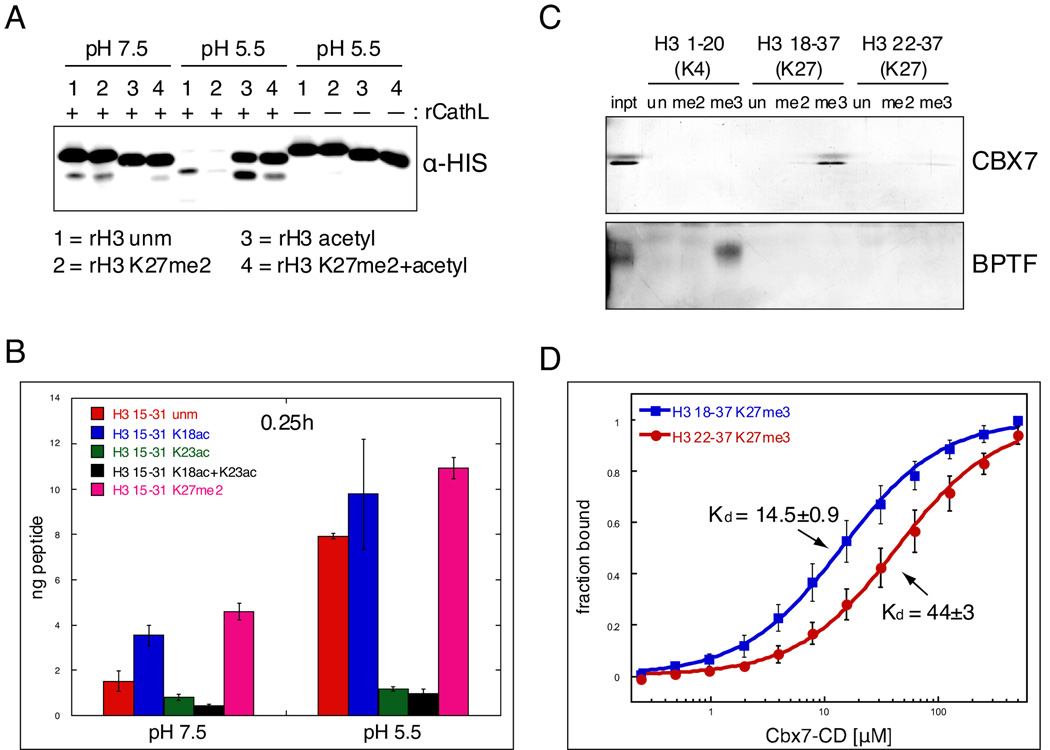
(A) rCathepsin L was incubated with each of four rH3 substrates: 1= rH3 unmodified, 2= rH3 alkylated to K27me2, 3= rH3 pan-acetylated with acetic anhydride, 4= rH3+K27me2 pan-acetylated with acetic anhydride.
(B) H3 cleavage reactions were performed as in (A) using synthesized peptides that represent the H3 tail from amino acids 15 to 31. Reactions were incubated with ~250pmol peptide and quenched with 0.1% TFA before being plated in duplicate for ELISA with the H3cs.1 antibody. Signal was normalized to that of mock reactions for each peptide. Results represent the mean of three independent experiments ± SD.
(C) Peptide pull-down assays were performed with the chromodomain of mouse CBX7 and the PHD finger of human BPTF.
(D) Fluorescence anisotropy Cbx7-CD protein binding to non-cleaved peptide (18–37) vs. cleaved peptide (22–37); binding decreases 3 fold with cleaved peptide, p<0.01. Kds are in µM±SEM. Data points represent the mean±SD.