Abstract
Discarded tires are an important habitat for larvae of multiple species of disease-transmitting mosquitoes. Although tire locations likely influence composition and abundance of vectors, there are few data linking vector populations to the characteristics of the aquatic tire environment. We sampled water-filled tires at three times at a forested and an unforested site to evaluate how differences in detritus inputs or nutrients in these two macrohabitats may be associated with composition of mosquito-dominated invertebrate communities. The forested site had significantly greater inputs of leaves, twigs, seeds, and fine detritus at the first sampling, but subsequent sampling indicated no differences in inputs of any detritus type. Total phosphorous levels were significantly greater in the forested site, but there was no difference in total nitrogen or total ion concentrations during any sampling. Chlorophyll a levels were not different between sites, even though light levels were greater and canopy cover was less at the unforested site. Culex restuans dominated at the unforested site, and Ochlerotatus triseriatus, Anopheles barberi, and Orthopodomyia signifera were found primarily in the forest. Tires at the forested site had significantly more species but not more individuals than at the unforested site. Leaf amount was a good predictor of densities of Oc. triseriatus and overall abundance of mosquitoes in the forest, whereas the amount of seeds was a good predictor of overall invertebrate richness and of Oc. triseriatus numbers in the unforested site. Differences in mosquito assemblage composition between forested and unforested locations may be explained by greater inputs of plant-based detritus and some nutrients, but other factors, such as macrohabitat or host preferences of adult mosquitoes, also may be important.
Keyword Index: Chlorophyll a, Culex restuans, nutrients, macrohabitat, Ochlerotatus triseriatus
INTRODUCTION
In North America, water-filled discarded vehicle tires support larvae of important mosquito vectors of human and animal disease, including Aedes albopictus (Skuse), Culex pipiens (L.), Ochlerotatus triseriatus (Say, formerly in the genus Aedes, Reinert 2000), and Aedes aegypti (L.) (Haramis 1984, Livdahl and Willey 1991, Juliano 1998, Swanson et al. 2000, Aliabadi and Juliano 2002, Costanzo et al. 2005). These species have been implicated in the transmission of arboviral diseases, such as dengue, La Crosse encephalitis, eastern equine encephalitis, and West Nile encephalitis (Mitchell et al. 1993, Ibanez-Bernal et al. 1997, Turell et al. 2001, Gerhardt et al. 2001, Turell et al. 2005). Illegal dumping of tires in urban and wooded areas, coupled with declines in natural mosquito breeding sites (e.g., tree holes), have made discarded tires an important source of disease vectors. Tires in peridomestic areas have attracted attention because they are a major source of biting mosquitoes, and thus can be significant for human health (Costanzo et al. 2005). Moreover, it is likely that importation of tires from Asia was the means by which Aedes albopictus was introduced into North America (Hawley et al. 1987). Work in Florida and Brazil has documented that mosquito species in tires are differentially distributed across urban-rural gradients (O’Meara et al. 1995, Swanson et al. 2000, Braks et al. 2004), whereas others have shown that forested-unforested gradients can affect the community of mosquitoes found within tires (Beier et al. 1983, Haramis 1984, Lampman et al. 1997, Joy et al. 2003). Although populations of mosquitoes have been measured across different habitat types, little information exists about variation in detritus types or amounts or dissolved nutrient levels across these same habitats. Understanding the associations of habitat gradients with populations and community composition of mosquitoes may be important for understanding disease transmission in different kinds of habitats and may aid in planning more effective vector control strategies.
Organic detritus, particularly leaves, is thought to be the major organic carbon source for communities of container mosquito larvae (Merritt et al. 1992, Kaufman et al. 2002) as most forms of primary productivity are absent (Carpenter 1983). Growth and survival of container mosquitoes are affected by detritus type and dissolved nutrients (e.g., Walker et al 1997, Léonard and Juliano 1995, Paradise and Dunson 1997, Yee and Juliano 2006). Although detritus inputs and their effect on mosquitoes in tree holes have been investigated (e.g., Lounibos et al. 1993, Léonard and Juliano 1995, Walker et al. 1997, Yee2), little is known about detrital inputs into discarded vehicle tires, how such inputs affect populations and communities of invertebrate, or species interactions that may shape these communities. For example, laboratory data suggest that the invasive mosquito Aedes albopictus should out-compete the native mosquito Oc. triseriatus in discarded tires, whereas coexistence should occur in natural tree holes (Livdahl and Willey 1991). One hypothesis for this outcome is that tires contain resources that are absent in tree holes and better exploited by Ae. albopictus (Livdahl and Willey 1991), although there are no data quantifying such resources in tires. Understanding the patterns of larval production from aquatic habitats is critical for understanding processes affecting adult populations (Gimnig et al. 2001).
Our research goal was to describe the patterns of composition of mosquito-dominated invertebrate communities and to test for differences in detritus and nutrient concentrations in a set of tires in a forested and an unforested area. Our work poses two questions about community composition between the forested and unforested sites: 1) How do tires in a forested and a nearby unforested site differ in invertebrate community composition? and 2) What, if any, differences in detritus type and amount or nutrients may be associated with observed community or population differences between the forested and unforested sites? Differences in detritus inputs seem likely, as forested and unforested locations often differ in tree cover, incident light, and disturbance.
MATERIALS AND METHODS
We sampled two sites with numerous tires, one forested and one unforested, approximately 24 km apart, on 5 and 27 July, and 5 September of 2004. Sampling was conducted at these times to quantify larvae hatching from eggs laid during the current season, rather than larvae hatching from eggs laid the previous fall. The unforested site was located in Chenoa, IL, (approximately 40° 44′ 43″ N, 88° 43′ 21″ W) at an automobile repair facility. The majority of tires at this location were haphazardly stacked or placed upright in a large uncovered pile on one side of a building. Other tires were scattered in an open grassy area and in a refuse pile near several small trees. Tree cover was absent for all but a few of these tires. All tires sampled were within a 30 m × 30 m area and were often moved and occasionally removed for recycling. Because of this, we kept track of tire movement by marking them with white paint. Human presence was almost continuous across sampling dates.
The forested site was located at Parklands Merwin Preserve, Lexington, IL (40° 39′ 10″ N, 88° 52′ 21″ W). Each tire rested upright against a tree in a heavily wooded, largely undisturbed area. A dense canopy of mixed mature hardwoods (e.g., white oak, red elm, sugar maple) covered this site. Because of their location, these tires received little human contact, although blood-meal hosts like white-tailed deer (Odocoileus virginianus Boddaert), small mammals, and birds were abundant in the area. These tires had been used previously to test effects of habitat drying on invertebrate communities (Van Rhein et al. 2000) but had been undisturbed for at least three years prior to our sampling.
At each site, we randomly chose 20 tires of similar size with standing water to sample. We randomly designated half of these tires at each site for quantification of litter and invertebrates, whereas the other ten tires were used for measurement of dissolved nutrients. For the litter tires, we removed the entire contents of the tire and sorted organic detritus into five categories: twigs (including bark), leaves, seeds (including fruit), fine (including soil and sand), and animal detritus (dead invertebrates). All detritus was dried at 50°C for ≥ 48 h and weighed to the nearest 0.0001 mg using a Cahn microbalance (Cahn Instruments, CA). The first sample (5 July) enabled us to quantify long-term detritus accumulation, but subsequent samples (27 July, 5 Sept) quantified short-term detritus additions. In addition to detritus, we removed, counted, and identified to species or morphospecies all macroinvertebrates, based on consistent morphological differences. From this we calculated total abundance and total species richness for each tire. Mosquito pupae were excluded from analyses. Any tire that did not refill with rain water between sampling dates was used to quantify detritus amounts, but could not be used for invertebrate sampling. Invertebrates were not replaced, so data from the latter two sampling periods would represent recolonization of these tires.
For the other ten tires at each site, we removed only water samples, allowing invertebrates and detritus to remain undisturbed over the sampling period. For all sampling dates, we removed approximately 110 ml of water from each tire for measurements of conductivity (total ion concentration, μMHOS cm−1), total nitrogen (TN) (mg liter−1), and total phosphorus (TP) (mg liter−1). We determined TN and TP at each sample using a Hach D800 Colorimeter with appropriate kits (Hach Inc., CO). Conductivity values were obtained using a Cole-Parmer conductivity meter. These nutrients have been shown to be related to the size or performance of populations of container mosquitoes (Beier et al. 1983, Yee and Juliano 2006, Yee and Juliano 2006, 2007). On 27 July and 5 September we also removed an additional 100 ml sample for quantification of water-column algae via measurements of chlorophyll a (mg m−3). We have observed algae in tires, and algae can be important for the nutrition of some mosquito species (Hedeen 1953, Kaufman et al. 2006). Each sample was filtered through a 47 mm circular glass microfiber filter in a PALL magnetic GF/F filter funnel, and stored in a sealed opaque film canister at 5° C until we processed the samples. Samples were processed following Clesceri et al. (1989). Tires that dried between sampling dates could not be used for nutrient and chlorophyll a samples.
Measurements of canopy cover and light levels were taken at sites in the summer of 2005 (14 July, 15 August, 17 September) approximately at the same time as sampling of detritus, invertebrates, and nutrients in 2004. We assumed any differences in canopy cover between sites were similar in those two years. At each site, canopy cover was measured using a spherical densitometer, with the final reading representing the mean of values obtained at each cardinal direction above each tire. Canopy cover values range from 0 (100% cover) to 39 (0% cover). Light readings were obtained using a Panlux electronic light meter (Gossen Photo, Nürnberg) and were recorded in foot candles (fc). All readings at each site were taken above previously sampled tires (unforested site) or at trees where sampled tires had been located (forested site).
Statistical analyses
Differences in amounts of each detritus type at each site for each date were analyzed using multivariate analysis of variance (MANOVA, PROC GLM, SAS Institute Inc., 2004). Significant MANOVA effects were interpreted using standardized canonical coeffcients (Scheiner 2001), which quantify the magnitude of the contributions of the individual dependent variables to significant multivariate differences. Data for detritus amounts were transformed (log(x+1)) to meet assumptions of normality and homogeneity of variances.
To reduce the number of correlated variables for invertebrate abundances, we used principal component analysis (PCA) (PROC FACTOR, SAS Institute Inc., 2004) to summarize invertebrate community structure in tires across all sampling dates for both sites. We retained principal components (PCs) with eigenvalues ≥ 1.0 (Hatcher 1994). Differences among tires between sites for each date were analyzed using MANOVA on PC scores, again using standardized canonical coeffcients to interpret significant MANOVA effects. We also tested for differences in the total species and total abundance per tire between sites using MANOVA. Variables were transformed (log(x+1)) to meet assumptions of normality and homogeneity of variances.
We determined if detritus amounts of each type (twig, leaf, seed, fine, animal) were good predictors of community and population variables at each site, separately, using stepwise multiple regression analysis (PROC REG, SAS Institute Inc., 2004). We chose to analyze only the most common mosquito species (Cx. restuans, Oc. triseriatus, Table 1), total abundance and richness of all mosquitoes, and the total abundance and richness of all invertebrates.
Table 1.
Mean amounts (g) (± 1 SE) of different detritus types in discarded vehicle tires (n = 10) in a forested and unforested site across three sampling dates.
| Twig | Leaf | Seed | Fine | Animal | ||
|---|---|---|---|---|---|---|
| Date | Site | |||||
| 5 Jul 2004 | Unforested | 0.005 ± 0.005 | 0.003 ± 0.002 | 0.000 ± 0.000 | 2.22 ± 1.19 | 0.030 ± 0.021 |
| Forested | 7.216 ± 1.847 | 24.471 ± 5.396 | 6.510 ± 2.175 | 35.881 ± 11.813 | 0.001 ± 0.001 | |
| 27 Jul 2004 | Unforested | 0.111 ± 0.068 | 0.298 ± 0.221 | 0.013 ± 0.013 | 0.894 ± 0.281 | 0.006 ± 0.003 |
| Forested | 0.960 ± 0.423 | 0.883 ± 0.375 | 0.407 ± 0.164 | 6.511 ± 1.820 | 0.002 ± 0.001 | |
| 5 Sep 2004 | Unforested | 0.012 ± 0.009 | 0.047 ± 0.025 | 0.000 ± 0.000 | 0.567 ± 0.423 | 0.052 ± 0.040 |
| Forested | 0.095 ± 0.050 | 0.177 ±0.052 | 0.102 ± 0.091 | 1.538 ± 0.797 | 0.002 ± 0.001 |
MANOVA was used to test for differences between sites in nutrient values (TN, TP, conductivity, and chlorophyll a) for each month separately. We did not measure chlorophyll a during the first sampling period nor TP during the last sampling period. All variables were transformed (log(x+1)) to meet assumptions of normality and homogeneity of variances. We used two-tailed, unequal variance t-tests to determine if canopy cover or light levels differed between the two sites within each of the three sample periods.
RESULTS
There were significant differences in amounts of each type of detritus between the two sites in early July (Pillai’s Trace5, 14 = 0.928, P < 0.001), but not for late July (Pillai’s Trace5, 11 = 0.360, P = 0.356) or September (Pillai’s Trace5, 11 = 0.396, P = 0.283). Amounts of all detritus types decreased at both sites after the first sample. For early July, there were significant differences between sites in plant and fine detritus, but not animal detritus (Table 1), with tires in the forested site containing more twigs, leaves, seeds, and fine detritus. Standardized canonical coeffcients (SSC) indicated that differences in leaf detritus (SSC = 2.43) contributed more to differences between sites than other detritus types (SSC twigs = 0.71, seeds = 0.10, fine = 0.54). Although MANOVA yielded no difference in detritus types in late July, we did detect significant univariate differences (P < 0.05) between sites in seeds and fine detritus, with the forested site having more of each (Table 1). Detritus amounts did not differ in September.
Eleven species of invertebrates inhabited tires during our sampling, with four species of culicids representing about 45% of all individuals collected (Table 2). Principal component analysis of invertebrate communities yielded 4 PCs that explained 65.3% of the variation in invertebrate abundances. PC1 (eigen value = 2.87, 26% of the variation) yielded positive scores for tires with high abundances of Ochlerotatus triseriatus, Anopheles barberi (Coquillett), and Culicoides sp., and negative scores for tires with high abundances of Culex restuans (Theobald). PC2 (eigen value = 1.71, 16% variation explained) yielded high positive scores for tires with high abundances of Helodes sp. and the syrphid Mallota posticata (F.), and negative scores for tires with few of these species. PC3 (eigen value = 1.45, 13% variation explained) yielded positive values for tires with high abundances of Chironomidae sp. A and negative scores for tires with high abundances of Chironomidae sp. B. Finally, PC4 (eigen value = 1.43, 10% variance explained) yielded positive scores for tires with high abundances of Orthopodomyia signifera (Coquillett) and Telmatoscopus sp. and negative scores for tires with high abundances of Ostracods.
Table 2.
Invertebrates collected from tires across three sampling dates in 2004 at an unforested (U) and forested (F) site. Numbers of individuals during each of three surveys at each site (n = 6 to 10 tires per site).
| 5 July | 27 July | 5 Sept | ||||||
|---|---|---|---|---|---|---|---|---|
| Site: | U | F | U | F | U | F | Totals | |
| Family or Group
|
Species
|
|||||||
| Culicidae | Culex restuans | 732 | 15 | 643 | 0 | 141 | 75 | 1,606 |
| Ochlerotatus triseriatus | 0 | 130 | 17 | 536 | 20 | 485 | 1,188 | |
| Anopheles barberi | 0 | 15 | 0 | 38 | 0 | 14 | 67 | |
| Orthopodomyia signifera | 0 | 5 | 0 | 8 | 0 | 5 | 18 | |
| Syrphidae | Mallota posticata | 0 | 1 | 0 | 5 | 0 | 7 | 13 |
| Chironomidae | Unknown sp. A | 0 | 5 | 95 | 283 | 142 | 63 | 588 |
| Unknown sp. B | 497 | 0 | 0 | 0 | 5 | 0 | 502 | |
| Ceratopogonidae | Culicoides sp. | 0 | 50 | 0 | 99 | 0 | 0 | 149 |
| Psychodidae | Telmatoscopus sp. | 0 | 5 | 1 | 1 | 0 | 1 | 8 |
| Scirtidae | Helodes sp. | 0 | 0 | 0 | 54 | 0 | 10 | 64 |
| Ostracoda | Unknown | 195 | 207 | 194 | 970 | 81 | 194 | 1,841 |
| Totals | 1,415 | 439 | 950 | 1,994 | 389 | 854 | 6,041 | |
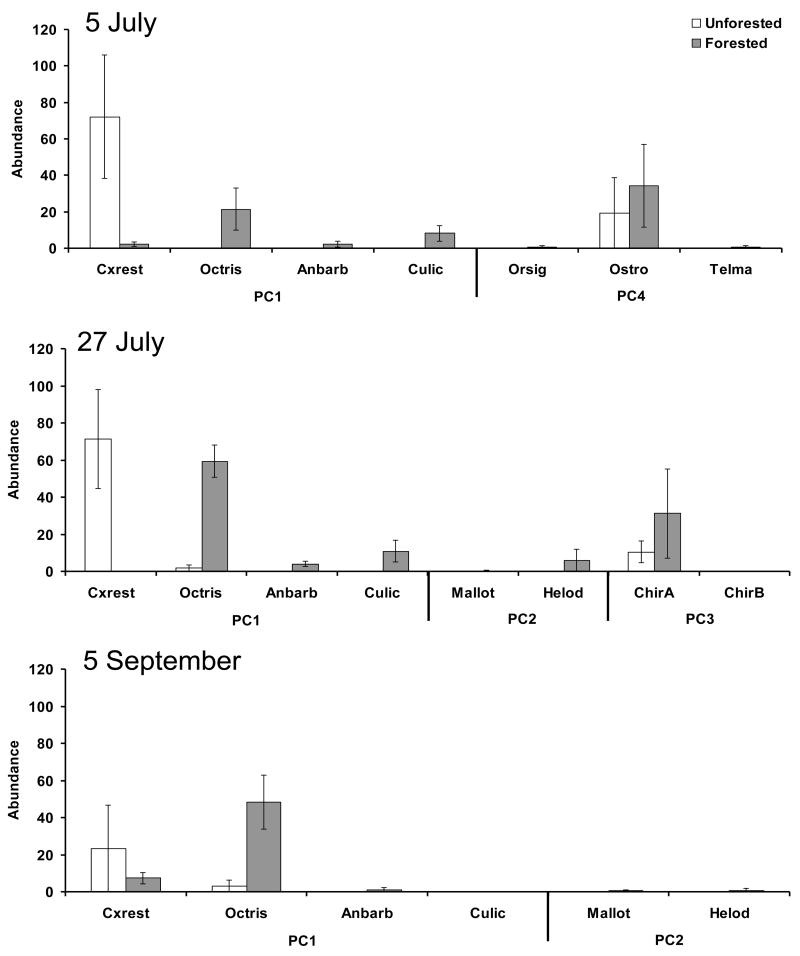
There were significant differences in the invertebrate community between sites during all sampling periods (Table 3, Figure 1). Specifically, differences in sites in early July depended mostly on PC1 and PC4 (Table 3), with tires in the unforested site having high numbers of Culex restuans, but tires in the forested site having high numbers of Ochlerotatus triseriatus, Anopheles barberi, Culicoides sp, Orthopodomyia signifera, and Telmatoscopus sp. (Table 3, Figure 1). Tires in late July in the unforested site also had high numbers of Cx. restuans, whereas tires in the forested site were characterized by communities containing high numbers of Oc. triseriatus, An. barberi, Culicoides sp, Chironomidae sp. A, and Helodes sp. (Table 3, Figure 1). In the final sample, tires in the forested habitat had high numbers of Oc. triseriatus, An. barberi, Mallota posticata, and Helodes sp., whereas tires in the unforested site had high numbers of Cx. restuans (Table 3, Figure 1).
Table 3.
Results of MANOVA testing for differences between sites (Forested, Unforested) in invertebrate communities for three sampling dates. Large (bold) standardized canonical coeffcients (SSC) indicate principal component axes (PC) which are important in explaining significant multivariate effects. PCs are the same across dates. Species and signs listed under PC indicate which abundances load most heavily on each PC.
| Date | 5 July | 27 July | 5 September |
|---|---|---|---|
| Pillai’s Trace (df, df) P-value | 0.725 (4, 11) 0.004 | 0.858 (4, 13) < 0.001 | 0.557 (4, 11) 0.046 |
| PC | SSC | SCC | SCC |
| 1: + Oc. triseriatus, +An. barberi + Culicoides sp. − Cx. restuans | 1.836 | 2.639 | 1.213 |
| 2: + Mallota posticata, + Helodes sp. | − 0.337 | 0.949 | 0.764 |
| 3: + Chironomidae sp. A −Chironomidae sp. B | 0.472 | 0.922 | −0.299 |
| 4: + Telmatoscopus sp. + Or. signifera − Ostracoda | 0.764 | − 0.048 | −0.068 |
Figure 1.
Mean abundance (± 1 SE) per tire of invertebrate species collected across dates at a forested and unforested site. Bars represent the mean of 6–10 tires at each site per sampling date. Species associated with principal component (PC) axes which were important in explaining differences in invertebrate communities (Table 3) between sites are presented along the x-axis. Species abbreviations are: Cx. restuans (Cxrest), Oc. triseriatus (Octris), An. barberi (Anbarb), Culicoides sp. (Culic), Or. signifera (Orsig), Ostracoda (Ostra), Telmatoscopus sp. (Telma), Chironomidae sp. A (ChirA), Chironomidae sp. B (ChirB), Mallota posticata (Mallot), and Helodes sp. (Helod).
MANOVA revealed that total richness contributed more to the significant difference on 5 July (Pillai’s Trace2, 13 = 0.817, P < 0.001) than did total abundance, with more species in the forested (mean = 4.50 ± 0.44) than in the unforested site (mean = 1.10 ± 0.44). On 27 July, we obtained a similar result (Pillai’s Trace2, 15 = 0.547, P = 0.003), with richness contributing more than abundance to the significant differences between sites. Richness was higher in the forested (3.89 ± 0.25) than in the unforested site (2.00 ± 0.33). No differences were apparent for richness or abundance on 5 September (Pillai’s Trace2, 13 = 0.226, P = 0.194).
Multiple regression analysis revealed that abundance of Oc. triseriatus increased with increasing amounts of seeds in the unforested site, whereas abundance of this species declined with increasing leaf litter in the forested site (Table 4). Total culicidae abundance displayed also a negative relationship to leaf litter (Table 4). Abundance of Cx. restuans were invariant with any input of detritus (Table 4). Richness (unforested) of all mosquitoes increased with increasing leaf litter, although total abundance of all invertebrates did not change with changes in any detritus type (Table 4). Total species richness increased as amounts of seeds rose in the unforested site, but did not change with any detritus type in the forested site (Table 4).
Table 4.
Multiple regression results testing for the relationship between amount of different detritus types and community (richness = S) or population measures (total abundance = N) in tires.
| Habitat | Dependant Variable | df error | Variable | R2 | slope ± SE | P |
|---|---|---|---|---|---|---|
| Unforested | Oc. triseriatus | 22 | Seeds | 0.596 | 116.23 ± 20.40 | < 0.001 |
| Cx. restuans | 22 | none | – | – | – | |
| Culicidae S | 22 | Leaves | 0.168 | 0.544 ± 0.251 | 0.047 | |
| Culicidae N | 22 | none | – | – | – | |
| Total S | 22 | Seeds | 0.163 | 18.09 ± 8.75 | 0.051 | |
| Total N | 22 | none | – | – | – | |
| Forested | Oc. triseriatus | 23 | Leaves | 0.143 | −0.892 ± 0.455 | 0.062 |
| Cx. restuans | 23 | none | – | – | – | |
| Culicidae S | 23 | none | – | – | – | |
| Culicidae N | 23 | Leaves | 0.199 | − 1.02 ± 0.425 | 0.025 | |
| Total S | 23 | none | – | – | – | |
| Total N | 23 | none | – | – | – |
Nutrient levels in tires were significantly different between sites in early July (Pillai’s Trace3, 9 = 0.556, P = 0.053) and late July (Pillai’s Trace4, 10 = 0.849, P = 0.004), but were not different in September (Pillai’s Trace3, 12 = 0.241, P = 0.329, Table 5). Overall, phosphorous levels, which were significantly greater in the forested site than in the unforested site, was the primary contributor to difference between sites (largest SSC). Other variables did not differ between sites (Table 5).
Table 5.
Mean (± 1 SE) nutrient levels in discarded vehicle tires in a forested (F) and unforested (U) site. Chlorophyll a was not measured in early July and TP was not measured in September.
| Date | Site | Nitrogen (TN) (mg L−1) | Phosphorous (TP) (mg L−1) | Conductivity (μMHOS cm−1) | Chlorophyll a (mg m−3) |
|---|---|---|---|---|---|
| 5 Jul | U | 2.02 ± 0.63 | 1.44 ± 0.67 | 1833.20 ± 546.26 | — |
| F | 2.00 ± 1.35 | 12.99 ± 8.42 | 523.67 ± 260.15 | — | |
| 27 Jul | U | 0.80 ± 0.28 | 2.30 ± 0.88 | 973.40 ± 311.31 | 78.74 ± 24.03 |
| F | 0.67 ± 0.21 | 12.73 ± 0.21 | 420.17 ± 79.77 | 33.69 ± 14.47 | |
| 5 Sep | U | 1.42 ± 0.83 | — | 456.00 ± 118.22 | 63.78 ± 40.34 |
| F | 1.83 ± 0.45 | — | 265.83 ± 53.98 | 12.06 ± 8.87 |
Light levels and canopy cover were significantly different between sites across dates (P < 0.05) and light levels on 14 July were nearly significant (P = 0.062). Light levels were greater at the unforested site (July = 1,724.3, August = 780.1, September = 1055.9 fc) than at the forested site (July = 69.3, August = 24.0, September = 13.8 fc). Canopy cover was less at the unforested site (July = 18.3, August = 17.2, September = 20.5) than in the woods (July = 1.5, August = 1.4, September = 1.4) on all dates.
DISCUSSION
The two sites we sampled were markedly different in their mosquito-dominated invertebrate community, as well as in measures of detritus type and amount and in one water nutrient variable. Thus, our hypothesis that the aquatic environment within tires would differ between these paired forested and unforested sites is supported. Moreover, differences in detritus amounts were associated with differences in invertebrate communities within tires and were of some predictive power in explaining abundance of the second most common mosquito, Ochlerotatus triseriatus, and abundance and richness of all mosquitoes. Thus, these data suggest that some of the variation in mosquito populations between sites may be attributable to variation in detritus inputs into tires. Tree holes represent another mosquito-dominated aquatic system that is based on allochthonous inputs of terrestrial detritus (Daugherty et al. 2000, Kitching 2001, Yee 2006), and where inputs of detritus type and amount affect community and populations of invertebrates (Jenkins et al. 1992, Srivastava and Lawton 1998, Yee and Juliano 2007).
Masses of detritus types were different during the first sampling date, but amounts on subsequent dates were statistically indistinguishable. The first sample represented detritus accumulations which could have occurred over a long time period, especially in the forest (> 3 years). The age of the tires at the unforested site is unknown, but they are likely to be younger than the forest, due to frequent movement of tires for recycling. Surprisingly, we found no difference between sites in inputs between the first and second or the second and third sampling, suggesting that input rates were similar across this time of the year. Autumn inputs of senescent leaves, which would have occurred after our sampling had ended, would have caused our tires in the forested site to receive substantial inputs of leaves and other plant-derived detritus. Animal detritus, which has been shown to provide greater nutrition to container mosquito larvae (Yee and Juliano 2006), and which attracts more species and individuals to container habitats (Yee and Juliano 2007), did not differ between sites on any date (Table 1). Because animal detritus decomposes at a faster rate than plant-derived detritus (Yee and Juliano 2006), and because of the relatively long time between our surveys, additions of animal detritus to these tires may be underestimated.
Algal abundances, as measured by chlorophyll a, did not differ between sites on any date, even though tires in the unforested site had much higher light levels than did those in the unforested site. The amount of algae in tires may have been depressed by feeding by invertebrates, particularly mosquito larvae, which could have obscured chlorophyll a levels between sites. Ostracods, in particular, also may have impacted chlorophyll a levels as this group preferentially feeds on algae (Thorp and Covich 2001), although higher densities of this group in the forest seem to provide evidence against this conjecture. Light level alone may not be a good predictor of algal abundance, as tire orientation or placement among other tires is likely to affect the amount of sunlight reaching the aquatic environment. Beier et al. (1983) made a qualitative assessment of algae in shaded and unshaded tires and found large differences in the amount of algae, but found that algal abundance was not correlated with mosquito species abundance. Gut content analysis of a variety of pond and flood-plain associated mosquito larvae show that algae can be an important food source (Merritt et al. 1992, Wallace and Merritt 1999, Kaufman et al. 2006), although the degree to which algae benefits container mosquitoes is unknown.
Of the three nutrients we measured, only total phosphorous differed between sites (Table 4). Because we did not measure ambient TP, TN, or conductivity from tires in which we sampled invertebrates or detritus, we could not correlate directly these nutrients with invertebrate abundance or community composition. Phosphorous limitation may occur in many different aquatic systems (Schindler 1977, Sundareshwar et al. 2003) and can affect the growth of many organisms, including Daphnia (Elser et al. 2001, Makino et al. 2002) and many insect larvae (Perkins et al. 2004). The relatively high levels of TP relative to TN encountered in these tires suggests nitrogen limitation. Addition of detritus-derived nitrogen has been shown to be important for mosquito growth (Carpenter 1982, Walker et al. 1991, 1997, Kaufman et al. 2002, Yee and Juliano 2006). Besides nitrogen, labile carbon is much more likely to limit production of basal microbial food resources than is phosphorous in some mosquito systems (Kaufman et al. 2002, Kaufman and Walker 2006). Nutrient limitation for mosquitoes or their microorganism food base in tires is largely unexplored.
Densities of Oc. triseriatus were over 30 times higher in the forested than in the unforested site, a result consistent with this species’ preference for wooded areas (Wilton 1968, Beier et al. 1983, Haramis 1984, Copeland and Craig 1989, Lampman et al. 1997, Joy and Hildreth-Whitehair 2000, Barker et al. 2003). The association of Oc. triseriatus with forested areas may be the result of macrohabitat preference by adults, resulting from differences in the quality or quantity of blood meal hosts or resting sites, but it may also be the result of resources for larvae found within containers. We observed a negative association between Oc. triseriatus abundance and leaf litter levels in the forested site (Table 4), suggesting that large amounts of leaf litter may have negative effects on larval population through the leaching of high tannin levels (Mercer 1993, Sota 1993) or a high detritus to water ratio. In those cases, high detrital loads may raise levels of toxins generated through microorganism decomposition or may prevent larvae access to the surface for breathing. Water volume has been suggested to be more important to populations of Oc. triseriatus than litter amounts (Paradise 2004) because water volume may be indicative of habitat stability (Sota et al. 1994). Even though abundance of this species was greater in the forested site, we did encounter a few individuals in the unforested site (Table 1). The positive association between seeds and Oc. triseriatus in the unforested site is perhaps misleading, as there was only a single tire that contained seeds across all sampling dates, although this tire contained 43% of the individuals found at this site.
In contrast to Oc. triseriatus, populations of Culex restuans were almost exclusively found in the unforested site (Table 1). In fact, densities of these two species showed a significant negative correlation across tires (r = − 0.33, P = 0.021). Cx. restuans abundance was not related to detritus types (Table 4). Experimental evidence suggests that Culex spp. females oviposit in containers with high organic concentrations (Beehler et al. 1994, Reiskind and Wilson 2004), but other factors such as the presence of conspecific larvae (Reiskind and Wilson 2004) can affect patterns of oviposition. Because we do not have data for nutrient loads and larval abundance for the same tires, it remains possible that high nutrient concentrations are important as an attractant to ovipositing females. Cx. restuans has been suggested to be an early colonizer of new tires (Berry and Craig 1984, Andreadis 1988), such as tires located at service stations and tire dealerships (Baumgartner 1988) where tire turnover is high. Populations of Cx. restuans in shaded tires have been shown to respond to water color and turbidity, which has been hypothesized to be related to detritus quantity (Beier et al. 1983). Our data suggest that detritus amount was not an important factor explaining the density of Cx. restuans larvae, although water color or turbidity may be more indicative of microorganism food sources for larvae than detritus amounts per se.
Other species of mosquitoes we encountered are commonly encountered in tree holes (Orthopodomyia signifera, Hanson et al. 1995, Anopheles barberi, Copeland and Craig 1989), although their relatively low abundances in this study suggest that tires may act as a secondary habitat. Both Or. signifera (Hanson et al. 1995) and An. barberi (Copeland and Craig 1989) appear to require oviposition stimuli associated with plant-derived detritus, such as tannins. Abundances of Oc. triseriatus and An. barberi were positively correlated (r = 0.53, P < 0.001), even though they have been shown to be negatively correlated across tree holes within forested areas (Bradshaw and Holzapfel 1983). Late instar An. barberi are facultative predators on early instar Oc. triseriatus larvae (Petersen et al. 1969), so a positive relationship may simply indicate the association of predators with their prey.
Non-mosquito invertebrates in tires often have been ignored, as most of these species are not major vectors of human or animal disease. We found that the community of non-mosquitoes is similar to that found in tree holes at the forested site (Yee 2006, Yee and Juliano 2007), and within vehicle tires at other Illinois sites (Yee, unpublished data). Tree holes in this area of Illinois contain a suite of non-mosquito invertebrates including scirtid beetles, the syrphid Mallota posticata, ceratopogonids in the genus Culicoides, and moth flies in the genus Telmatoscopus, although densities of all these species were far lower than those observed in tree holes (Yee and Juliano 2007). Low densities of scirtid beetles in tires contrast with high numbers encountered in tree holes (Daugherty and Juliano 2001). The wood lining of tree holes may promote greater microorganism growth for surface scraping scirtids, although container volume and leaf litter volumes are also important for sciritid abundance (Daugherty and Juliano 2001). Both chironomids and ostracods were abundant in tires at both sites (Table 2), which is perhaps a reflection of the tendency of these groups to occupy a wide variety of aquatic habitats (Thorp and Covich 2001).
Our study documents differences in the fauna associations with discarded vehicle tires in forested and unforested habitats, and found that detritus inputs may be important in explaining variation in populations of some mosquito species and in overall community patterns. The fact that communities remained somewhat distinct between sites even after we removed detritus from tires suggests that recolonization was in part due to effects of the surrounding habitats (e.g., detritus inputs, temperature, light, source pool). Because the community of invertebrates found in the forested site was similar to communities found in tree holes, we hypothesize that source pools of colonists from tree holes contribute highly to the communities of invertebrates in tires in or near forests. Tires found outside of forested locations may also contain tree hole species, although they are more likely to contain species that are habitat generalists (e.g., Culex restuans). Unfortunately, many human-associated habitats are likely to be unforested and thus contain abundant Culex species that may pose health risks.
Acknowledgments
We thank K. Kirkham for assistance with analysis of chlorophyll a, T. Kling, E. Stone, and C. Villanueva provided assistance with collection and processing of samples. is project was supported by a grant to S. A. Juliano and D. A. Yee from the National Institute of Allergy and Infectious Disease (R15-AI-051374).
Footnotes
Yee, D.A. 2006. Effects of species interactions and productivity on aquatic macroinvertebrate diversity and community composition in tree holes: patterns and mechanisms. Ph.D. dissertation, Illinois State University.
REFERENCES CITED
- Aliabadi BK, Juliano SA. Escape from gregarine parasites affects the competitive impact of an invasive mosquito. Biol Invas. 2002;4:283–297. doi: 10.1023/A:1020933705556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis TG. A survey of mosquitoes breeding in used tire stockpiles in Connecticut. J Am Mosq Contr Assoc. 1988;4:256–260. [PubMed] [Google Scholar]
- Barker CM, Brewster CC, Paulson SL. Spatiotemporal oviposition and habitat preference of Ochlerotatus triseriatus and Aedes albopictus in an emerging focus of LaCrosse virus. J Am Mosq Contr Assoc. 2003;19:382–391. [PubMed] [Google Scholar]
- Baumgartner DL. Surburban accumulations of discarded tires in northeasten Illinois and their associated mosquitoes. J Am Mosq Contr Assoc. 1988;4:500–508. [PubMed] [Google Scholar]
- Beehler JW, Miller JG, Mulla MS. Filed evaluation of synthetic compounds mediating oviposition in Culex mosquitoes (Diptera, Culicidae) J Chem Ecol. 1994;20:281–291. doi: 10.1007/BF02064436. [DOI] [PubMed] [Google Scholar]
- Beier JC, Patricoski C, Travis M, Kranzfelder J. Influence of water chemical and environmental parameters on larval mosquito dynamics in tires. Environ Entomol. 1983;12:434–438. [Google Scholar]
- Berry WJ, Craig GB., Jr Bionomics of Aedes atropalpus breeding in scrap tires in northern Indiana. Mosq News. 1984;44:476–484. [Google Scholar]
- Bradshaw WE, Holzapfel CM. Predator-mediated, non-equilibrium coexistence of tree-hole mosquitoes in southeastern North America. Oecologia. 1983;57:239–256. doi: 10.1007/BF00379586. [DOI] [PubMed] [Google Scholar]
- Braks MAH, Honório N, Lounibos LP, Lourenço-De-Oliveira R, Juliano SA. Interspecific competition between two invasive species of container mosquitoes, Aedes aegypti and Aedes albopictus (Diptera: Culicidae), in Brazil. Ann Entomol Soc Am. 97:130–139. [Google Scholar]
- Carpenter SR. Stemflow chemistry: effects on population dynamics of detrivorous mosquitoes in tree-hole ecosystems. Oecologia. 1982;53:1–6. doi: 10.1007/BF00377128. [DOI] [PubMed] [Google Scholar]
- Carpenter SR. Resource limitation of larval treehole mosquitoes subsisting on beech detritus. Ecology. 1983;64:219–223. [Google Scholar]
- Clesceri LS, Greenberg AE, Trussell RR. Standard methods for the examination of water and wastewater. American Public Health Association - American Water Works Association - Water Pollution Control Federation; Washington D.C: 1989. [Google Scholar]
- Copeland RS, Craig GB., Jr Winter cold influences the spatial and age distributions of the North American treehole mosquito Anopheles barberi. Oecologia. 1989;79:287–292. doi: 10.1007/BF00384306. [DOI] [PubMed] [Google Scholar]
- Costanzo KS, Mormann K, Juliano SA. Asymmetrical competition and patterns of abundance of Aedes albopictus and Culex pipiens (Diptera: Culicidae) J Med Entomol. 2005;42:559–570. doi: 10.1093/jmedent/42.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty MP, Alto BM, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2000;37:364–372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty MP, Juliano SA. Factors affecting the abundance of scirtid beetles in container habitats. J N Am Benthol Assoc. 2001;20:109–117. [Google Scholar]
- Elser JJ, Hayakawa K, Urabe J. Nutrient limitation reduces food quality for zooplankton: Daphnia response to seston phosphorus enrichment. Ecology. 2001;82:898–903. [Google Scholar]
- Gimnig JE, Ombok M, Kamau L, Hawley WA. Characteristics of larval Anopheline (Diptera: Culicidae) habitats in Western Kenya. J Med Entomol. 2001;38:282–288. doi: 10.1603/0022-2585-38.2.282. [DOI] [PubMed] [Google Scholar]
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, Panella NA, Powell EE, Nasci RS. First isolation of LaCrosse virus from naturally infected Aedes albopictus. Emerg Infect Dis. 2001;37:807–811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Novak RJ, Lampman RL, Vodkin MH. Notes on the biology of Orthopodomyia in Illinois. J Am Mosq Contr Assoc. 1995;11:375–376. [PubMed] [Google Scholar]
- Haramis LD. Aedes triseriatus: a comparison of density in tree holes vs. discarded tires. Mosq News. 1984;44:485–489. [Google Scholar]
- Hatcher L. A step-by-step approach to using the SAS system for factor analysis and structural equation modeling. SAS Institute; Cary, NC: 1994. [Google Scholar]
- Hawley WA, Reiter P, Copeland RS, Pumpuni CB, Craig GB., Jr Aedes albopictus in North America: Probable introduction in used tires from northern Asia. Science. 1987;236:1114–1116. doi: 10.1126/science.3576225. [DOI] [PubMed] [Google Scholar]
- Hedeen RA. The biology of the mosquito Aedes atropalpus Colquillet. J Kans Entomol Soc. 1953;26:1–10. [Google Scholar]
- Ibanez-Bernal BBS, Mutebi JP, Argot E, Rodriguez G. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med Vet Entomol. 1997;11:305–309. doi: 10.1111/j.1365-2915.1997.tb00413.x. 1997. [DOI] [PubMed] [Google Scholar]
- Jenkins B, Kitching RL, Pimm SL. Productivity, disturbance and food web structure at a local spatial scale in experimental container habitats. Oikos. 1992;65:249–255. [Google Scholar]
- Joy JJ, Hildreth-Whitehair A. Larval habitat characterization for Aedes triseriatus (Say), the mosquito vector of LaCrosse encephalitis in West Virginia. Wilder Environ Med. 2000;11:79–83. doi: 10.1580/1080-6032(2000)011[0079:lhcfat]2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Joy JJ, Hanna AA, Jennedy BA. Spatial and temporal variation in the mosquitoes (Diptera: Culicidae) inhabiting waste tires in Nicholas County, West Virginia. J Med Entomol. 2003;40:73–77. doi: 10.1603/0022-2585-40.1.73. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Kaufman MG, Goodfriend W, Kohler-Garrigan A, Walker ED, Klug MJ. Soluble nutrient effects on microbial communities and mosquito production in Ochlerotatus triseriatus habitats. Aquat Microbiol Ecol. 2002;29:73–88. [Google Scholar]
- Kaufman MG, Wanja E, Maknojia S, Bayoh MN, Vulule JM, Walker ED. Importance of algal biomass to growth and development of Anopheles gambiae larvae. J Med Entomol. 2006;43:669–676. doi: 10.1603/0022-2585(2006)43[669:ioabtg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kaufman MG, Walker ED. Indirect effects of soluble nitrogen on growth of Ochlerotatus triseriatus larvae in container habitats. J Med Entomol. 2006;43:677–688. doi: 10.1603/0022-2585(2006)43[677:ieosno]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kitching RL. Food webs in phytotelmata: “bottom-up” and “top-down” explanations for community structure. Annu Rev Entomol. 2001;46:729–760. doi: 10.1146/annurev.ento.46.1.729. [DOI] [PubMed] [Google Scholar]
- Lampman R, Hanson S, Novak R. Seasonal abundance and distribution of mosquitoes at a rural waste tire site in Illinois. J Am Mosq Contr Assoc. 1997;13:193–200. [PubMed] [Google Scholar]
- Léonard PM, Juliano SA. Effect of leaf litter and density on fitness and populations performance of the tree hole mosquito Aedes triseriatus. Ecol Entomol. 1995;20:125–136. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Nishimura N, Escher RL. Fitness of a treehole mosquito: influences of food type and predation. Oikos. 1993;66:114–118. [Google Scholar]
- Makino W, Urabe J, Elser JJ, Yoshimizu C. Evidence of phosphorus-limited individual and population growth of Daphnia in a Canadian Shield lake. Oikos. 2002;96:197–205. [Google Scholar]
- Mercer DR. Effect of tannic acid concentration on development of the western tree hole mosquito, Aedes sierrensis (Diptera: Culicidae) J Chem Ecol. 1993;19:1119–1127. doi: 10.1007/BF00987373. [DOI] [PubMed] [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Ann Rev Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ, McLean RG, Nasci RS, Crans WJ, Smith GC, Caccamise DF. Susceptibility parameter of Aedes albopictus to per oral infection with Eastern equine encephalitis virus. J Med Entomol. 1993;30:233–235. doi: 10.1093/jmedent/30.1.233. [DOI] [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Jr, Gettman AD, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Paradise CJ. Relationship of water and leaf litter variability to insect inhabiting treeholes. J North Am Bentho Soc. 2004;23:793–805. [Google Scholar]
- Paradise CJ, Dunson WA. Effects of water cations on treehole insect communities. Ann Entomol Soc Am. 1997;90:798–805. [Google Scholar]
- Perkins MC, Woods HA, Harrison JF, Elser JJ. Dietary phosphorus affects the growth of larval Manduca sexta. Arch Insect Biochem Physio. 2004;55:153–168. doi: 10.1002/arch.10133. [DOI] [PubMed] [Google Scholar]
- Petersen JJ, Chapman JC, Willis OR. Predation of Anopheles barberi Coquillett on first instar mosquito larvae. Mosq News. 1969;29:134–135. [Google Scholar]
- Reinert JF. New classification for the composite genus Aedes (Diptera, Culicidae, Aedini), elevation of subgenus Ochlerotatus to generic rank reclassification of the other subgenera, and notes on certain subgenera and species. J Am Mosq Contr Assoc. 2000;16:175–188. [PubMed] [Google Scholar]
- Reiskind MH, Wilson ML. Culex restuans (Diptera: Culicidae) oviposition behavior determined by larval habitat quality and quantity in Southeastern Michigan. J Med Entomol. 2004;41:179–186. doi: 10.1603/0022-2585-41.2.179. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS/STAT 9.1 User’s Guide. SAS Institute Inc; Cary, NC: 2004. [Google Scholar]
- Scheiner SM. MANOVA. Multiple response variables and multi species interactions. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. 2. Oxford University Press; Oxford: 2001. pp. 99–133. [Google Scholar]
- Schindler DW. Evolution of phosphorus limitation in lakes. Science. 1977;195:260–262. doi: 10.1126/science.195.4275.260. [DOI] [PubMed] [Google Scholar]
- Sota T. Performance of Aedes albopictus and A. riversi larvae (Diptera: Culicidae) in waters that contain tannic acid and decaying leaves: is the treehole species better adapted to treehole water. Ann Entomol Soc Am. 1993;86:450–457. [Google Scholar]
- Sota T, Mogi M, Hayamizu E. Habitat stability and the larva mosquito community in treeholes and other containers on a temperate island. Res Pop Ecol. 1994;36:93–104. [Google Scholar]
- Srivastava DS, Lawton JH. Why more productive sites have more species: an experimental test of theory using tree-hole communities. Am Nat. 1998;152:510–529. doi: 10.1086/286187. 1998.Whymoreproductive. [DOI] [PubMed] [Google Scholar]
- Sundareshwar PV, Morris JT, Koepffer EK, Fornwalt B. Phosphorus limitation of coastal ecosystem processes. Science. 2003;299:563–565. doi: 10.1126/science.1079100. [DOI] [PubMed] [Google Scholar]
- Swanson J, Lancaster M, Anderson J, Crandell M, Haramis L, Grimstad P, Kitron U. Overwintering and establishment of Aedes albopictus (Diptera: Culicidae) in an urban La Crosse virus enzootic site in Illinois. J Med Entomol. 2000;37:454–460. doi: 10.1093/jmedent/37.3.454. [DOI] [PubMed] [Google Scholar]
- Thorp JH, Covich AP. Ecology and classification of North American freshwater invertebrates. Academic Press; NY: 2001. [Google Scholar]
- Turell M, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American Mosquitoes (Diptera: Culicidae) for West Nile Virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O’Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera : Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Van Rhein SL, Flanary BE, Juliano SA. Effects of habitat type and drying on Ascogregarina barretti (Eugregarinida: Lecudinidae) infection in Aedes triseritatus (Diptera: Culicidae) J Med Entomol. 2000;37:950–956. doi: 10.1603/0022-2585-37.6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology. 1991;72:1529–1546. [Google Scholar]
- Walker ED, Kaufman MG, Ayres MP, Riede MH, Merritt RW. Effects of variation in quality of leaf detritus on growth of the eastern tree-hole mosquito, Aedes triseriatus (Diptera: Culicidae) Can J Zool. 1997;75:706–718. [Google Scholar]
- Wallace JR, Merritt RW. Influence of microclimate, food, and predation on Anopheles quadrimaculatus (Diptera: Culicidae) growth and development rates, survivorship, and adult size in a Michigan pond. Environ Entomol. 1999;28:233–239. [Google Scholar]
- Wilton DP. Oviposition site selection by the tree-hole mosquito, Aedes triseriatus (Say) J Med Entomol. 1968;5:189–194. doi: 10.1093/jmedent/5.2.189. [DOI] [PubMed] [Google Scholar]
- Yee DA, Juliano SA. Consequences of detritus type in an aquatic microsystem: assessing water quality, micro-organisms, and the performance of the dominant consumer. Fresh Biol. 2006;51:448–459. doi: 10.1111/j.1365-2427.2005.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Juliano SA. Abundance matters. A field experiment testing the More Individuals Hypothesis for richness-productivity relationships. Oecologia. 2007 doi: 10.1007/s00442-007-0707-1. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]