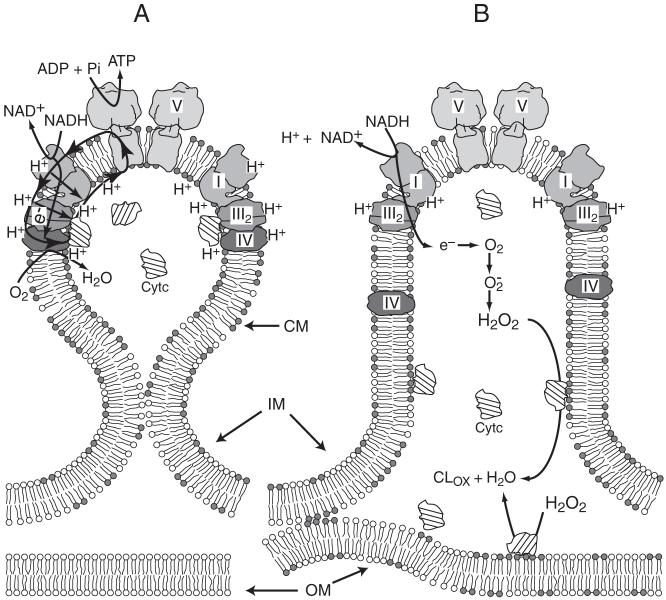
Fig. 8.5.
Model for involvement of CL in the energy coupling processes or apoptosis in mitochondria. (A) Under normal conditions cristae membranes (CM) are separated from the remaining mitochondrial inner boundary membranes (IM) by the cristae junctions. CM are composed of microdomains enriched in CL (depicted in the bilayer as dimer of gray headgroups) and Complexes I, III, IV and V (F1F0ATP synthase) organized in I1III2IV1 (see Fig. 2.4) and V2 supercomplexes. The proton gradient (positive outward) generated by electron transfer from NADH to molecular oxygen via the supercomplex I1III2IV1 (with channeling of cytochrome c (Cytc) between Complex III and IV) is utilized to synthesize ATP. CL participates in the trapping of protons promoting localized protonic coupling between the respiratory supercomplex and Complex V located in close proximity to each other (figure adapted from (Papa et al., 2006)). (B) During early stages of apoptosis cristae junctions open allowing more equal distribution of CL between CM and IM with significant appearance of CL in the outer membrane (OM) via the contacts sites between the latter two membranes. Reduction of CL in the CM favors delocalization of the proton gradient and partial dissociation of Complex IV from the supercomplex. Binding of cytochrome c to CL disrupts electron flow between Complexes III and IV and induces increased production of reactive oxygen species by Complex III. Interaction of cytochrome c with CL converts the protein into a peroxidase, which catalyzes CL peroxidation (CLox), which further reduces CL levels (Basova et al., 2007)