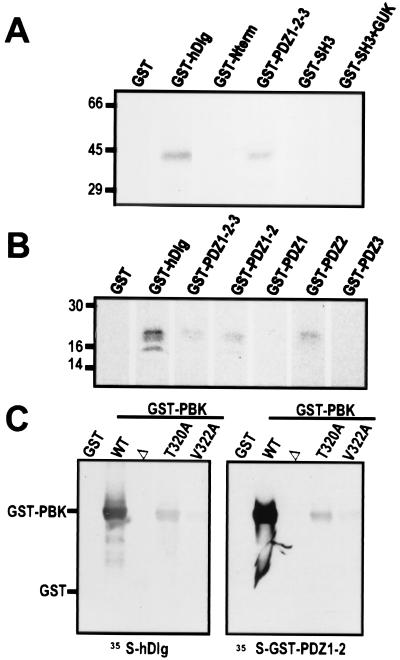
Figure 3.
The C-terminal ETDV motif of PBK interacts specifically with PDZ2 of hDlg in vitro. (A and B) Autoradiographs of in vitro binding assays. (A) 14C-labeled PBK (43 kDa) coprecipitated with bead-bound GST fusions of full-length hDlg (GST-hDlg) and of the three PDZ domains of hDlg (GST-PDZ1–2-3). PBK did not bind to GST or fusions of GST with the N-terminal, SH3, or guanylate kinase homology domains of hDlg (GST-N-term, GST-SH3, and GST-SH3 + GUK). (B) Radiolabeled C terminus of PBK (20kDa) bound to GST-fusion proteins containing PDZ2 of hDlg (GST-hDlg, GST-PDZ1–2-3, GST-PDZ1–2, and GST-PDZ2) but not to fusions of the other PDZ domains of hDlg (GST-PDZ1, GST-PDZ3). (C) Autoradiograph of blot overlay assays. GST fusions of PBK with wild-type C-terminal motif (WT), with a deletion of the last 10 amino acids (Δ), or with single residue substitutions within the C-terminal motif (T320A and V322A) were separated on a gel and transferred to nitrocellulose. Blots were probed with radiolabeled hDlg (Left) or with a radiolabeled GST fusion of PDZ1–2 of hDlg (Right).