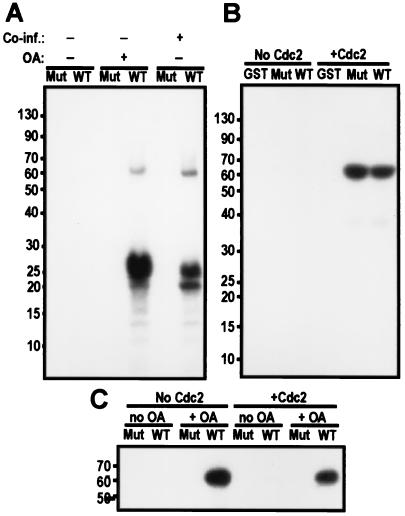
Figure 9.
cdc2/cyclin B phosphorylation is important but not sufficient to activate PBK. (A) Autoradiograph of an in vitro kinase assay blot with GST fusions of PBK (WT) or a mutant of PBK (K64A-K65A, Mut) purified from cell treated or not with okadaic acid and coinfected or not with cdc2/cyclin B. Wild-type PBK isolated from cells treated with okadaic acid or from cells also expressing human cdc2/cyclin B can phosphorylate histone. (B) Autoradiograph of the blot of an in vitro kinase assay where GST and GST fusions of PBK (WT) or a mutant of PBK (K64A-K65A, Mut) purified from Sf9 cells not treated with okadaic acid were used as substrates for cdc2/cyclin B. Both wild-type and mutant PBK GST fusions are substrates of cdc2/cyclin B, but GST alone is not. (C) Autoradiograph of an autophosphorylation assay. GST fusions of PBK (WT) or a mutant of PBK (K64A and K65A, Mut) were purified from Sf9 cells treated (+ OA) or not treated (no OA) with 100 nM okadaic acid before lysis. These fusion proteins were phosphorylated or not by cdc2/cyclin B by using nonradiolabeled ATP. The GST-PBK fusions were then isolated by using glutathione-Sepharose beads and washed several times. PBK was finally assayed for autophosphorylation activity in the presence of radiolabeled ATP, separated on a 10% tricine gel and transferred to nitrocellulose.