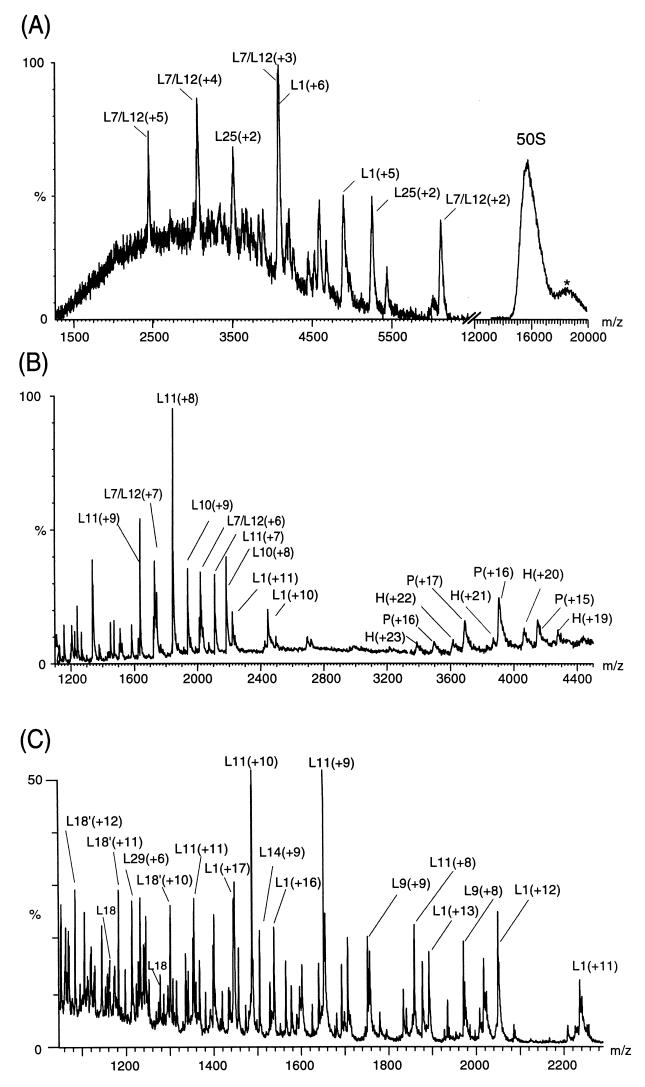
Figure 3.
Controlled dissociation of the 50S subunit (A) at low collision energy, (B) in the absence of collisional damping processes, and (C) under high energy conditions. The latter is from the 50S subunits from which the stalk complex had been removed before analysis. The peaks arising from individual proteins are labeled with their charge states in brackets. The spectra were obtained with a high cone voltage on the LCT (A), in the absence of argon gas in the collision cell and under low vacuum conditions in the quadrupole on the Q-ToF (B), and with no pressure control on the Platform (C) (14). The peak at m/z 18,500, labeled *, is attributed to the complementary fragment after dissociation of monomeric proteins from the intact 50S subunit whereas P and H are assigned to the pentamer, comprising of two copies of L7/L12 and L10, and the hexamer with the addition of protein L11. The peaks labeled L18′ in C correspond in mass to posttranslational modification of the protein L18. The protein L7 is N-acetyl L12 and although the species has been resolved previously (10, 14) no distinction is made in the spectra shown here because of the wide m/z range that has been displayed.