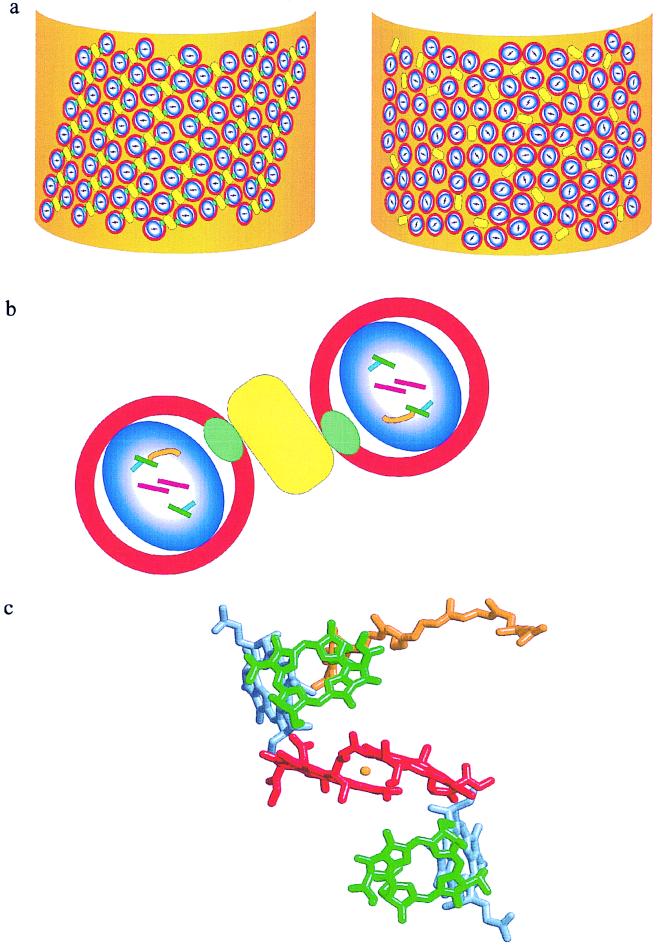
Figure 4.
A model of the organization of RC-LH1 core complexes in a membrane fragment of an LH2-minus mutant of Rb. sphaeroides. The core complexes in the left-hand representation contain PufX whereas those on the right do not. (a) The intracytoplasmic membrane is depicted as a membrane fragment in which the circular RC-LH1-PufX cores (blue and white oval enclosed by red circle) and cytochrome bc1 complex (ES) (yellow rectangle) are embedded. The long-range order of these complexes with respect to the long axis (y) of the membrane fragment is apparent when the PufX protein (green oval) is present in the core complex, whereas the cores that contain only RC-LH1 can adopt any orientation. The Qy transitions of the special pair of bacteriochlorophylls (P) are represented by the bars within the blue and white oval. (b) An enlarged view of a pair of core complexes flanking one or two cytochrome bc1 complexes, as suggested by Jungas et al. (16). The RC pigments P are in red, B in green, and H in blue, and the position of QB is marked by the orange curve. The bars represent the Qy transitions of these pigments, approximated by drawing a line between the NB and ND atoms within the macrocycles. (c) The pigments P, B, and H plus QB and the nonheme iron from the crystal structure of Rb. sphaeroides (Protein Data Bank ID code 1PCR) viewed along the C2 axis of symmetry. The color coding is as used in b with the nonheme iron represented by the orange sphere.