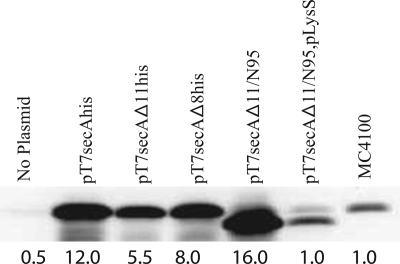
FIG. 1.
SecAΔ11/N95 is functional in vivo due to overproduction of SecA protein. BL21.19 containing the indicated plasmids was grown in LB supplemented with appropriate antibiotics at 30°C to an A600 of 0.2, and then it was shifted to 42°C for an additional 2 h. The A600 of all cultures were adjusted to 1.0 by dilution of LB, and then cells were chilled, harvested by sedimentation at 4°C, and resuspended in sample buffer (2% sodium dodecyl sulfate, 125 mM Tris-HCl [pH 6.8], 5% 2-mercaptoethanol, 15% glycerol, 0.005% bromophenol blue) for analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by Western blotting with SecA antisera and visualization by enhanced chemiluminescence utilizing SuperSignal West Pico (Pierce) and a Syngene Gelbox system as described previously (17). Prior to sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis, the protein concentration in each sample was measured utilizing the Bradford assay, and equivalent amounts of total protein were loaded onto the gel. SecA levels were determined using a Syngene Gelbox system; the amount of SecA present in wild-type strain MC4100 lacking any plasmid was arbitrarily defined as 1.0, and the amounts determined are indicated under the lanes.