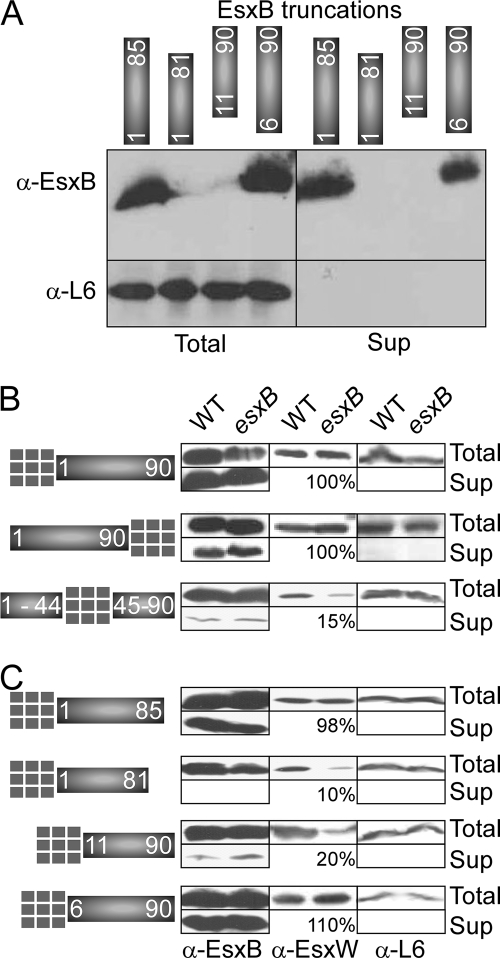
FIG. 7.
Sequence elements necessary for EsxB secretion. (A) Truncated variants of EsxB lacking either the first 5 and 10 or the last 5 and 9 amino acids at the N and C termini of the protein, respectively, were generated by cloning the corresponding DNA fragments in vector pOS1 carrying the pesxB. These variants are depicted as blocks of protein sequences consisting of residues 6 to 90, 11 to 90, 1 to 85, and 1 to 81. The plasmids were transformed in a mutant strain lacking esxB. Western blot analysis using antibodies against EsxB was used to examine complementation for EsxB production and secretion. The relevant part of the gel is shown. Longer exposure with chemiluminescence reagents did not reveal any truncated or degraded fragments for the EsxB proteins consisting of residues 11 to 90 or 1 to 81. (B and C) Secretion of protein hybrids. Ubiquitin was fused to full-length EsxB either at the N or C terminus or in the middle of the protein (B) or at the N terminus of the truncated variants shown in panel A. All clones were constructed using the same template and vector (pOS1 carrying pesxB; all cloning used the same restriction sites). Plasmids were transformed in wild-type strain Sterne (WT) or an isogenic mutant lacking esxB. Secretion profiles of hybrid proteins, EsxW, and ribosomal protein L6 (cytoplasmic control) were examined by Western blotting using appropriate antibodies. The amount of EsxW protein was evaluated by scanning autoradiogram. A value of 100% indicates no change in EsxW stability. Total, cell culture; Sup, supernatant; α, anti.