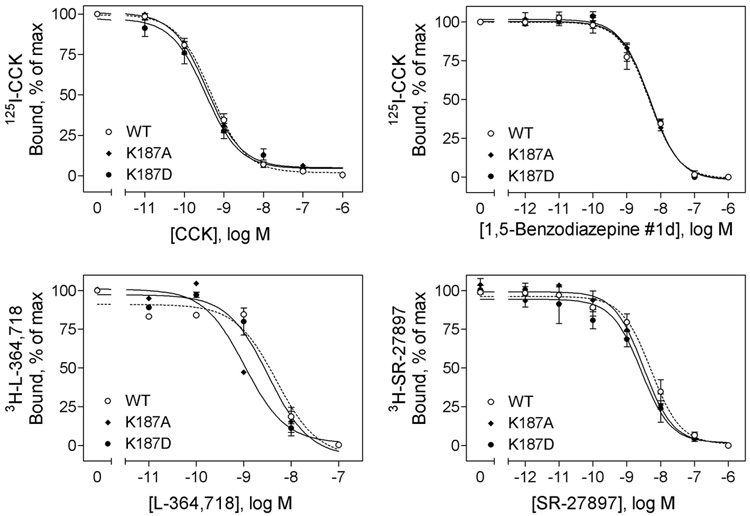
FIGURE 2.
Effects of mutation of CCK receptor residue Lys187 on binding of peptide and non-peptidyl ligands. Top left, curves for CCK to compete for binding of the 125I-CCK radioligand to membranes from CHO cells expressing the wild type, K187A, and K187D CCK receptor constructs. Top right, curves for non-peptidyl agonist, 1,5-benzodiazepine #1d, to compete for binding of the 125I-CCK radioligand to these cell membranes. Bottom left, curves for non-peptidyl antagonist, L-364,718, to compete for binding of the 3H-L-364,718 radioligand to these cell membranes. Bottom right, curves for non-peptidyl antagonist, SR-27897, to compete for binding of the 3H-SR-27897 radioligand to these cell membranes. Data are presented as means ± S.E.M. of data from a minimum of three independent experiments. Table 1 includes Ki and Bmax values for these series of studies. Mutation of CCK receptor residue Lys187 to either a neutral charge (Ala) or a reversed-charge (Asp) residue had no significant effects on ligand binding of either peptidyl agonist CCK or non-peptidyl agonist, 1,5-benzodiazepine #1d or antagonists, L-364,718 and SR-27897.