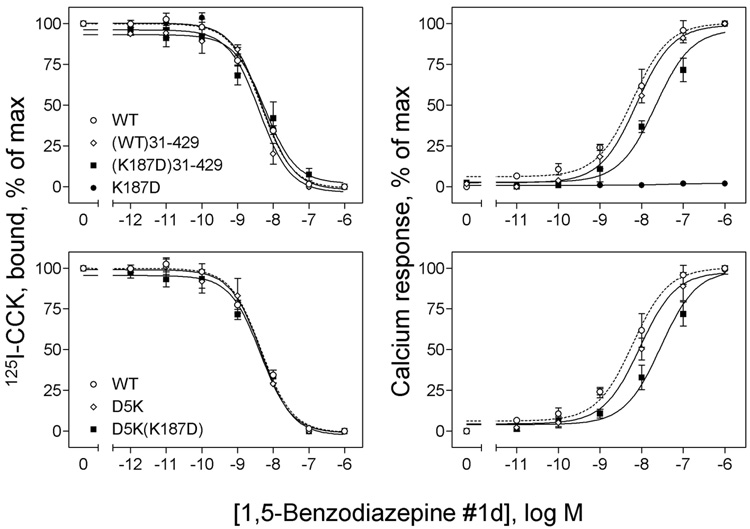
FIGURE 7.
Effects of truncation and site mutation of the amino terminus of the Lys187 mutant CCK receptor constructs on their non-peptidyl ligand binding and biological activity. Shown in left panels are curves for 1,5-benzodiazepine #1d to compete for binding of the 125I-CCK radioligand to membranes from CHO cells expressing the truncated WT ((WT)31–429) and K187D ((K187D)31–429) CCK receptor constructs (top) and membranes from COS cells expressing the site mutants, D5K and D5K(K187D) (bottom). As controls, data from WT and K187D are also shown. Ki and Bmax values are shown in Table 1. Shown in right panels are curves of intracellular calcium responses in these cells to increasing concentrations of 1,5-benzodiazepine #1d. Data are presented as means ± S.E.M. of data from a minimum of three independent experiments. Basal levels of intracellular calcium in CHO cells were similar for all the constructs (143 ± 21 nM), and maximal levels achieved after stimulation with 1,5-benzodiazepine #1d were 363 ± 49 (WT), 333 ± 29 ((WT)31–429) and 320 ± 33 ((K187D)31–429) nM. No significant differences in basal or maximal intracellular calcium levels were observed for any of the CCK receptor mutants expressed in COS cells. Basal levels of intracellular calcium were 160 ± 40 nM, and maximal levels achieved after stimulation with 1,5-benzodiazepine #1d were 262 ± 47 nM. These data demonstrate that amino-terminal truncation and site mutation of the K187D mutant CCK receptor are both able to restore intracellular calcium responses to 1,5-benzodiazepine #1d. Both constructs bound normally.