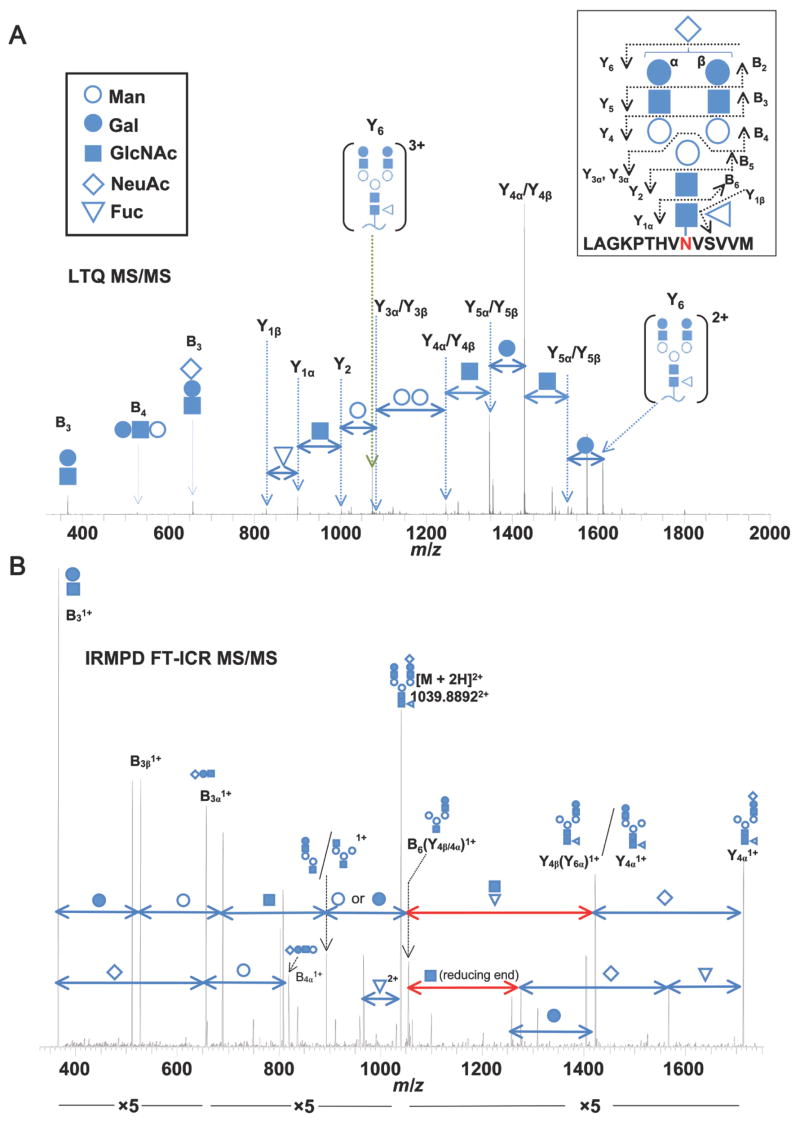
Figure 4. Representative LTQ tandem mass spectrum of an IgA1 N-glycopeptide.
(A) The collision-induced dissociation LTQ MS/MS spectrum confirms the structure of the IgA1 N-glycopeptide. Fragmentation of the IgA1 N-glycopeptide proceeds in at stepwise fashion from the terminal end of the N-glycan. The precursor mass in the FT-ICR MS spectrum (not shown) corresponded to the IgA1 tryptic peptide [L451-M464 + (NeuAc)1(Gal)2(GlcNAc)2(Fuc)1(Man)3(GlcNAc)2]3+. Fragment ions (3+ and 2+) corresponding to the loss of a single NeuAc are observed (Fragment ion Y6). From the Y62+ ion, the loss of subsequent monosaccharide residues can be observed down to the GlcNAc attached to the N459 confirming the expected N-glycan structure. Alternating fragments of the branched chains are observed (Y5 and Y4) followed by the loss of the core Man residues (Y3 and Y2). Additional fragments from the terminal end (B3, B4) further confirm the N-glycan structure. Symbols for the individual monosaccharide residues are defined in the key. Distinction between Gal and Man monosaccharides is assumed based on our monosaccharide compositional analysis and previously reported IgA1 N-glycan analysis (4, 52–54). Some individual N-glycan fragments are assigned to two possible bonds due to their isobaric composition. B) IRMPD FT-ICR MS/MS spectrum of the same IgA1 N-glycan shown in panel A, analyzed as a PNGase F-released glycan. In addition to the pattern of N-glycan fragments similar to those in A, monosaccharide losses which can only be attributed to the reducing end are observed (highlighted in red). The NeuAc residue is arbitrarily assigned to the β branch to simplify the labeled spectra.