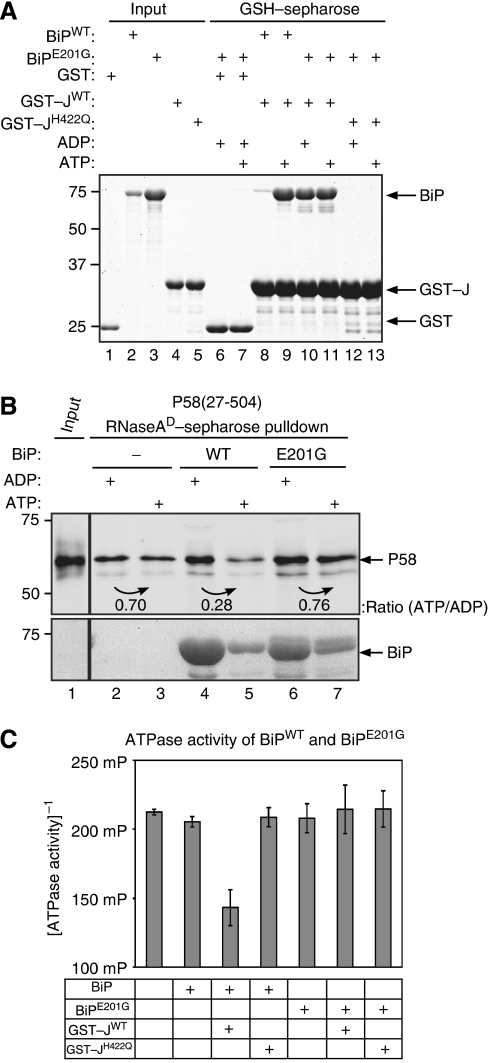
Figure 7.
The BiPE201G mutation separates J-domain binding from dissociation of P58 from denatured RNase A. (A) Coomassie-stained SDS–PAGE of BiP or BiPE201G mutant retained on glutathione sepharose beads to which GST, GST–JWT or GST–JH422Q had been pre-bound. Where indicated, the binding buffer was supplemented with ADP or ATP. Lanes 1–5 report on the input proteins used in the experimental lanes 6–13. (B) Immunoblot of bacterially expressed and purified P58 associated with denatured RNase A. Where indicated, the pre-formed complex was challenged with bacterially expressed BiPWT or BiPE201G (9 μM) in the presence of ADP or ATP. (C) ATPase activity of wild-type BiP and the E201G mutant alone or in the presence of GST–JWT or GST–JH422Q, measured by the conversion of ATP to ADP. The mean±s.e.m. of the fluorescence polarization signal is shown (as in Figure 6C).