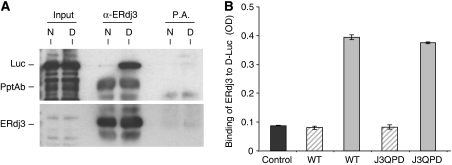
Figure 2.
WT and QPD ERdj3 bind to denatured luciferase similarly in vitro. (A) Temperature denatured (D) or native (N) luciferase (Luc) was directly loaded on a gel (first two lanes) or incubated with bacterially produced recombinant wild-type ERdj3 and then immunoprecipitated with either anti-ERdj3 polyclonal antiserum or with Protein A Sepharose beads alone. Reaction cocktails were subjected to reducing SDS–PAGE and then transferred to a PVDF membrane. The membrane was blotted with either goat anti-luciferase antiserum followed by donkey anti-goat Ig conjugated to HRP or with the polyclonal anti-ERdj3 followed by goat anti-rabbit Ig conjugated to HRP. In both cases, the signal was detected by chemiluminescence. (B) Chemically denatured luciferase (solid grey bars) or binding buffer alone (hatched bars) was used to coat 96-well plates. Recombinant wild-type or the QPD mutant ERdj3 proteins (0.5 μM) were added to the wells and bound ERdj3 was detected with a polyclonal anti-ERdj3 antiserum, followed by donkey anti-rabbit Ig conjugated to alkaline phosphatase. The DNTP substrate was added and after developing, the plates were read on a spectrophotometer and the signal was expressed in OD units. A luciferase-coated well that did not receive ERdj3 protein was treated similarly and serves as a negative control for the antibody (dark grey). All samples were run in triplicate and error bars are indicated.