Figure 6.
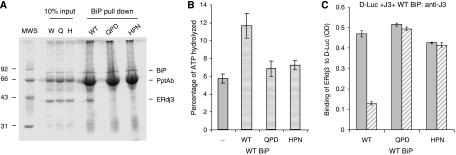
Wild-type BiP can only release wild-type ERdj3 from D-Luc. (A) Recombinant wild-type BiP (5 μg) was immunoprecipitated with a polyclonal anti-BiP antiserum and Protein A Sepharose beads. After washing, 20 μg of the indicated recombinant ERdj3 proteins was added to the BiP beads in ATPase buffer containing ATP. One-tenth of the reaction was removed for direct loading and the remaining nine-tenths were incubated at 4°C for 1 h. After washing, the samples were analysed by reducing SDS–PAGE and the gel was stained with Brilliant Coomassie Blue to visualize proteins. (B) ATPase assays were performed on wild-type BiP alone or with a four-fold molar excess of wild-type, HPN, or QPD ERdj3. ATP hydrolysis was measured by quantitating ADP and expressing it as a percentage of total nucleotide. (C) An experiment similar to that described in the previous figure was performed, except that either wild-type or mutant ERdj3 was bound to luciferase first. After washing, wild-type BiP was added with (hatched bars) or without (solid bars) ATP. The amount of ERdj3 that remained bound was detected with an anti-ERdj3 antiserum and expressed in OD units.