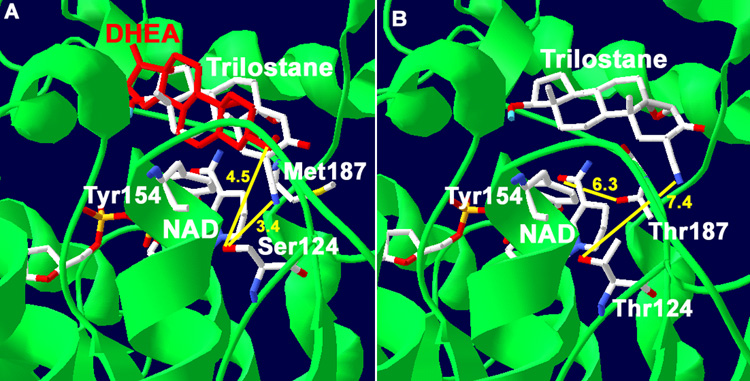
Fig. 1.
(A) Docking of trilostane with our structural model of human wild-type 3β-HSD1 shows the predicted interaction between the 2α-cyanogroup of trilostane and Ser124 residue of the wild-type enzyme (3.4 Å in yellow). The interaction of the 3β-hydroxyl group of substrate, DHEA, with Ser124 is also shown (4.5 Å yellow). The overlapping of DHEA (red) and trilostane is consistent with a competitive mode of inhibition. Met187 of 3β-HSD1 is shown to illustrate its lack of interaction with the nicotinamide group of NAD. (B) Docking of trilostane with the S124T mutant of 3β-HSD1 reveals a binding shift so that the 2α-cyanogroup of the inhibitor is 7.4 Å (yellow) from Thr124. In addition, the predicted interaction between the nicotinamide carbonyl of NAD and Thr187 of the M187T mutant enzyme (6.3 Å yellow) is shown. The position of the catalytic Tyr154 is labeled to identify the active site of the enzyme. The protein backbone (green), carbon (white), oxygen (red) and nitrogen (blue) atoms are indicated.