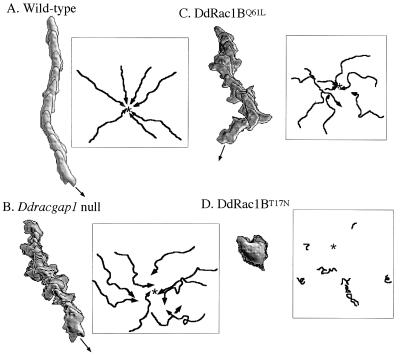
Figure 3.
Chemotactic movement of wild-type and mutant cells to a micropipette containing cAMP. Cells were pulsed with 30 nM cAMP at 6-min intervals for 5 h and cells were washed and resuspended in Na/KPO4 buffer containing 200 μM CaCl2 and MgCl2. A small volume of cells were plated on glass-bottomed microwell plates (MarTek, Ashland, MA) and allowed to adhere to the surface for approximately 20 min. A micropipette filled with 100 μm cAMP was positioned and images of chemotaxing cells were captured every 6 s. The movement of cells and changes in cell shape were analyzed with the dias program, a newly developed image analysis system. Superimposed images representing cell shape at 1-min intervals are shown. Movement of cells during chemotaxis was traced and is presented in boxes. Wild-type cells are very polarized, their migration is rapid and directed toward the tip of the micropipette, and the vast majority of pseudopodia are extended only in the direction of the micropipette. The majority of Ddracgap1 null cells move to the cAMP source, but they make many turns and lateral pseudopodia. Cells expressing DdRac1B61L show chemotactic defects similar to those of Ddracgap1 null cells. Cell migration is severely impaired in cells expressing DdRac1B17N. The star indicates the position of the cAMP source.