Abstract
Purpose
The purpose of this study was to examine the relationship of exercise energy expenditure (EEE) with both telomere length and telomerase activity in addition to accounting for hTERT C-1327T promoter genotype.
Methods
Sixty-nine (n = 34 males; n = 35 females) participants 50–70 yr were assessed for weekly EEE level using the Yale Physical Activity Survey. Lifetime consistency of EEE was also determined. Subjects were recruited across a large range of EEE levels and separated into quartiles: 0–990, 991–2340, 2341–3540, and 93541 kcalIwkj1. Relative telomere length and telomerase activity were measured in peripheral blood mononuclear cells (PBMC).
Results
The second EEE quartile exhibited significantly longer telomere lengths [1.12 T 0.03 relative units (RU)] than both the first and fourth EEE quartiles (0.94 T 0.03 and 0.96 T 0.03 RU, respectively; P G 0.05) but was not different from the third quartile. Telomerase activity was not different among the EEE quartiles. An association was observed between telomerase enzyme activity and hTERT genotype with the TT genotype (1.0 • 10j2 T 4.0 • 10j3 attomoles (amol) per 10,000 cells; n = 19) having significantly greater telomerase enzyme activity than both the CT (1.3 • 10j3 T 3.2 • 10j3; n = 30) and CC groups (5.0 • 10j4 T 3.9 • 10j3; n = 20; P = 0.01).
Conclusion
These results indicate that moderate physical activity levels may provide a protective effect on PBMC telomere length compared with both low and high EEE levels.
Keywords: TELOMERE BIOLOGY, EXERCISE ENERGY EXPENDITURE, hTERT GENOTYPE, GENETICS, DNA
Physical activity (PA) and increased physical fitness are known to decrease the likelihood of morbidity and mortality from a variety of causes (e.g., reduced cardiovascular disease (CVD), insulin resistance, and hypertension) (6), with concomitant increases in longevity (21). Whereas the reduction of disease end points will necessarily increase longevity, whether PA also directly affects cellular aging remains unclear for either rodents or humans (7). Telomere length is a primary biomarker of cellular aging that has recently been associated with CVD (1), insulin resistance and hypertension (14), and morbidity and mortality (9). In the present study, we explored the correlation of PA levels with telomere length and telomerase enzyme activity.
Telomeres are found on the ends of linear chromosomes and act as a mitotic clock (18), which shortens with every cell division until cellular senescence. Thus, telomeres are considered an important aging biomarker (2,4). Telomeres and their length are not, however, static entities but rather are a dynamic system (4). In certain cells, the ribonucleoprotein, telomerase, maintains and lengthens telomeres, allowing continued mitotic activity without progression to senescence (5). In cells with telomerase activity, telomere length can be maintained, thus delaying senescence and tissue aging. Human cells with telomerase activity include germ line cells, embryonic and stem cells, and adult proliferating cells such as those found in the immune system (19).
Recently, both telomere length and telomerase activity have been shown to be influenced by various environmental factors such as oxidative stress (14), psychological stress (16,17), and socioeconomic status (10). Collins et al. (13) found that athletes diagnosed with “fatigued athlete myopathic syndrome” (FAMS) had shorter muscle homogenate telomere length than age- and training-matched counterparts (13). Most recently, Cherkas et al. (11) found that leisure time PA was positively related to leukocyte telomere length in a dose-dependent manner, providing the first evidence of a role for typical PA in modifying telomere length.
In addition to environmental factors, the gene (TERT) that encodes for the catalytically active subunit of telomerase (human telomere reverse transcriptase; hTERT) is known to have a functional single nucleotide polymorphism (SNP) in the promoter region (C-1327T; rs2735940). The T allele of this SNP affects promoter activity of the TERT gene and has been associated with greater telomere length and greater telomerase activity compared with the C allele (22,23). Thus, hTERT genotype may play a moderating role in how environmental factors influence telomere biology.
The purpose of the present study was to explore the relationship of PA levels with telomere length and telomerase activity in the immune cells of older men and women. The association of the hTERT genotype with telomere length and telomerase enzyme activity was also investigated. We hypothesized that greater PA levels would be associated with longer telomere lengths and that individuals with the hTERT TT genotype would have longer telomere length and greater telomerase enzyme activity compared with the hTERT CC genotype carriers.
METHODS
Subjects
Seventy males and postmenopausal females aged 50–70 yr were recruited through newspaper advertisements, flyers, and word of mouth. One subject was excluded due to inconsistent PA responses, reducing the sample size to 69 for all analyses. All subjects were apparently healthy according to their responses to a medical health questionnaire. Potential volunteers were excluded if they had any underlying medical conditions, fatigue, acute myopathy, current infections, or treatment of cancer, as indicated on the medical health history questionnaire. If a subject had diagnosed heart disease, unmedicated hypertension, or previous blood or lymphocyte cancer, they were excluded due to the possible confounding effects of these conditions on the dependent variables. The University of Maryland Institutional Review Board approved the study, and all subjects provided written informed consent before participation.
Procedures
All subjects completed both a medical health history questionnaire and the Yale Physical Activity Survey (YPAS). The information extracted from the medical health history included date of birth, self-reported race and ethnicity, current medications, past and present medical disorders, self-reported PA consistency throughout past decades, and family history of age-related mental decline. Anthropometric measures, including height, weight, and body mass index (BMI), were collected using standard procedures.
Physical activity
Participants completed the interviewer-administered YPAS to estimate time spent in PA during a typical week during the past month (15). The YPAS has been tested for both reliability and validity, and the results have been found to be satisfactory (15,31). Two indices were gleaned from the information: 1) time spent in each specific YPAS activity was multiplied by an intensity code and was reported in kilocalories per minute and then 2) summed across all activities to create an index of total weekly energy expenditure reported in kilocalories per week. The two estimates were accompanied by the amount of time spent in each activity summed to provide a total time index reported in hours per week. Individuals were also asked to estimate the number of hours spent in five distinct physical dimensions (vigorous activity, leisurely walking, moving, standing, and sitting), each weighted differently. The frequency and duration of these activities were also recorded and scored, providing an index for each dimension (31). A summary index was calculated from the sum of all five indices.
On the basis of the results of the YPAS, total energy expenditure and exercise energy expenditure (EEE) were determined for each subject. EEE was extracted from the YPAS total energy expenditure (kcalIwkj1) data using only the exercise portion of the survey. In addition, the subject’s self-reported past 5-yr PA consistency as reported on the medical screening instrument was included in the PA assessment. Subjects responded to the question: “Over the past 5 years how consistent has your PA remained? A) Consistent, B) Somewhat consistent, C) Inconsistent.” Subjects were then asked to rate their PA level through life at ages 30–39, 40–49, 50–59, and 60–69 yr using the following scale: 1) very physically active (regular aerobic exercise and sports), 2) fairly physically active (sports and active leisure), 3) moderately physically active (hobbies, active leisure activities), 4) fairly physically inactive (very few sports, light physical leisure activities), and 5) very physically inactive (no sports, nonphysical leisure activities). These responses were coded and used as possible covariates in the statistical analyses. If a subject responded “Consistent” or “Somewhat consistent,” and the decade-to-decade PA level was consistent with the findings of the YPAS, the subject was included in the study. If the decade-to-decade PA level was inconsistent with the YPAS data, the subject was excluded from the study (only one subject was excluded on these grounds). The YPAS estimate of EEE in kilocalories per week (kcalIwkj1) was used as the primary independent variable.
Perceived stress survey
Subjects also completed a 10-question, 0- to 40-point scale Perceived Stress Survey (PSS). The PSS estimates the degree to which situations in one’s life are appraised as stressful (12). Because perceived psychological stress is known to affect the relationship of age and telomere length (16), the results of the survey were assessed as a possible covariate in the present study.
DNA isolation
All subjects had blood drawn from the antecubital vein using standard phlebotomy techniques. The blood was collected in standard 10-mL EDTA-treated vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ). Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation from each sample (Ficoll-Paque plus; Amersham Pharmacia Biotech, Piscataway, NJ). DNA was extracted from the PBMC using the PureGene DNA isolation system (Gentra Systems, Minneapolis, MN). The quantity and quality of the genomic DNA isolate were assessed by the Quant-iT PicoGreen dsDNA kit (Invitrogen, Carlsbad, CA). The integrity of random DNA isolates was evaluated by agarose gel electrophoresis.
Telomere length
Measurement of relative telomere lengths (Telomere PCR to Single-copy gene PCR or T/S ratio) was determined by quantitative real-time polymerase chain reaction (RT-PCR) as described by Cawthon (8) with the following modifications. The forward primer for the telomere PCR (T PCR) was tel1b [5¶-CGG TTT (GTTTGG)5 GTT-3¶] used at a final concentration of 125 nmolILj1. The reverse primer was tel2b [5¶-GGC TTG (CCTTAC)5 CCT-3¶] used at a final concentration of 312.5 nmolILj1. The forward primer for the single-copy gene (S PCR; acidic ribosomal phosphoprotein PO, 36B4) was RPLPO-F [5¶-CCC ATT CTA TCA TCA ACG GGT ACA A-3¶] used at a final concentration of 125 nmolILj1. The reverse primer was RPLPO-R [5¶-AGG TAG AAG GCC ACA TCA CC-3¶] used at a final concentration of 312.5 nmolILj1. SYBR Green Master mix (Applied Biosystems, Foster City, CA) was used and added to 8.75 KL of sample or reference DNA. Tubes containing 50, 25, 12.5, 6.25, and 3.125 ngIKLj1 of reference DNA were included in each PCR assay to determine the standard curve. All samples were run in triplicate. The assay was performed using the 7300 Real-Time PCR System (Applied Biosystems). The T PCR thermal cycling profile consisted of 10 min at 95-C followed by 30 cycles of 95-C for 15 s, 67-C for 1 min, 95-C for 15 s, followed by a dissociation stage of 95-C for 15 s, 60-C for 30 s, and 95-C for 15 s. The S PCR thermal cycling profile consisted of 10 min at 95-C followed by 40 cycles of 95-C for 15 s, 57-C for 1 min, 95-C for 15 s, followed by a dissociation stage of 95-C for 15 s, 60-C for 30 s, and 95-C for 15 s. The average intraplate coefficient of variation was 5.0% and the interplate coefficient of variation was also 5.0% for both the T and S PCR assays. The T PCR to S PCR ratio (T/S ratio) was derived from the Ct values of each unknown sample in each assay. The ratio of telomere repeat copy number to a single-copy gene copy number allowed the relative quantification of PBMC telomere length, which is proportional to the average telomere length, as described by Cawthon (8).
Telomerase
Telomerase enzyme activity was determined by a commercially available kit that uses the telomere repeat amplification protocol (TRAP; TRAPeze, Chemicon, NY) (16). The assay procedures followed the recommendations of the manufacturer, with each sample being analyzed in triplicate. The average intraplate coefficient of variation was 15.9%, and the interplate coefficient of variation was 15.8%.
Human telomere reverse transcriptase genotype
All subjects were genotyped for the C-1327T promoter polymorphism (rs2735940) in the TERT gene. The following primer sequences were designed for PCR: Forward hTERT-F—5¶-ACA GAC GCC CAG GAC CGC TCT-3¶; Reverse hTERT-R—5¶-CAG CGC TGC CTG AAA CTC-3¶. A guanine in the gene primer was replaced with a thiamine in the forward primer (underlined in forward primer sequence) to generate a novel restriction site, which allowed for the use of restriction digest methods to distinguish between the C and T alleles. The PCR was assembled with the following parameters: 2.5 KL of 10• PCR buffer with (NH4)2SO4 (Fermentas), 4.0 KL of 1.25 mmolILj1 dNTPs, 1.0 KL of 25 mmolILj1 MgCl2, 0.4 KL of 20 mmolILj1 hTERT-F, 0.4 KL of 20 mmolILj1 hTERT-R1, 0.2 KL of Taq polymerase (Fermentas), and 14.5 KL of dH2O to make a 25-KL reaction mixture. The thermal cycling conditions were as follows: 95-C for 5 min followed by a denaturation step (95-C for 30 s), annealing step (62-C for 30 s), and an extension step (72-C for 30 s) for 40 cycles followed by extension at 72-C for 5 min. The restriction enzyme EarI with T-allele specificity was purchased from New England Biolabs. After a 24-h digestion at 37-C, the products were visualized on a 3% agarose gel. The PCR fragment/band sizes were as follows for each genotype: TT = 225, CT = 202 and 225, and CC = 202. To verify the digest results, controls were generated via direct sequencing using the following sequencing primer: Forward hTERT-F-seq2–5¶-CAG AGC CTA GGC CGA TTC-3¶.
Statistics
All statistical procedures were performed using SAS version 9.1. All data were tested to ensure conformity with the assumptions of analysis of covariance (ANCOVA) testing, including normality and homoscedasticity. Relative telomerase enzyme activity neither was normally distributed nor did it have homogeneous variances; hence, a logarithm to base 10 transformation was performed for statistical analysis purposes. All statistical analyses were performed on the transformed telomerase data; however, nontransformed data are shown because these data are more biologically relevant. ANCOVA was used to test the association of hTERT genotype with both telomere length and telomerase activity, as well as with the 3 • 4 interaction with EEE (genotype • EEE quartile). The assumptions of ANCOVA and regression analysis were tested and observed to be satisfactory. Subjects were grouped into quartiles of EEE as follows for ANCOVA: 0–990, 991–2340, 2341–3540, and 93541 kcalIwkj1. ANCOVA was used to investigate the differences in telomere length and telomerase activity among EEE groups. If an ANCOVA model was statistically significant, specific contrasts were written to determine whether significant differences existed in telomere length and telomerase enzyme activity between specific EEE quartiles. Tukey’s HSD test was used for all post hoc comparisons. Significance was accepted at P e 0.05.
Statistical power
Sample size estimates were determined for telomere length (T/S ratios) and telomerase enzyme activity. The effect size ratio used for telomere length was a difference of 0.2 units, which has been significantly associated with psychological perceived stress (16) and with poor survival rate and increased mortality from heart and infectious disease (9). To obtain appropriate statistical power (A = 0.8; > = 0.05), at least 50 subjects needed to be included. The selected effect size for telomerase was 0.039 telomerase units, a difference of which has been associated with greater BMI and abdominal adiposity, higher systolic blood pressure, fasting glucose, LDL and total cholesterol, and total/HDL cholesterol ratio (17). To obtain appropriate power (A = 0.8; > = 0.05), at least 52 subjects needed to be included. Our final sample size was 69, adequate for 980% power for both telomere length and telomerase activity.
RESULTS
Subjects
Subject characteristics are shown in Table 1. Significant differences were observed among all groups for EEE, as expected (P G 0.001), with no other significant differences observed. Of the 70 recruited subjects, 1 was excluded on the basis of our PA inclusion criteria leaving 69 subjects for analysis.
TABLE 1.
Subject characteristics overall and by EEE quartile.
| N (F, M) |
||||||
|---|---|---|---|---|---|---|
| Overall (35, 34) | First (11, 6) | Second (12, 5) | Third (7, 8) | Fourth (5, 15) | P | |
| Age (yr) | 60.33 (4.9) | 59.17 T 1.2 | 61.41 T 1.2 | 58.53 T 1.2 | 62.15 T 1.1 | 0.08 |
| BMI (kgImj2) | 26.53 (4.5) | 27.38 T 1.2 | 26.41 T 1.2 | 25.24 T 1.2 | 26.02 T 1.1 | 0.62 |
| PSS | 10.52 (5.1) | 13.06 T 1.2 | 9.00 T 1.3 | 9.64 T 1.3 | 10.18 T 1.2 | 0.12 |
| EEE (kcalIwkj1) | 2697 (2309) | 312 T 283 | 1697 T 283* | 2763 T 301*† | 5526 T 261*†‡ | G0.01 |
| Total EE (kcalIwkj1) | 8094 (4218) | 5151 T 842 | 8007 T 842* | 6786 T 896 | 11,651 T 776*†‡ | G0.01 |
| Yale Summary index | 62.36 (24.46) | 41.53 T 4.9 | 58.76 T 4.9* | 68.06 T 5.3* | 78.85 T 4.6*†‡ | G0.01 |
| Yale Vigorous | 36.87 (15.8) | 14.11 T 4.2 | 27.94 T 4.2* | 42.67 T 4.5*† | 41.25 T 3.8*† | G0.01 |
Descriptive statistics is shown for the entire sample and by EEE quartiles. Values in the second column are reported as means (SD); all other values reported as least square means T SE.
Significantly different from the first EEE quartile (P G 0.05).
Significantly different from second EEE quartile (P G 0.05).
Significantly different from third EEE quartile (P G 0.05).
Telomere length
Subjects were grouped by quartiles of EEE (n = 17–18 per quartile). ANCOVA revealed a significant difference in telomere length among groups, with consistency of PA included as a covariate (P = 0.01; Fig. 1). As shown in Figure 1, the second EEE quartile exhibited significantly longer telomere lengths than both the first and fourth EEE quartiles (P = 0.001 and P = 0.04, respectively) but was not significantly different from the third EEE quartile. All other comparisons between groups were not significantly different. To address the possibility of sex differences, a sex-specific analysis was performed, which showed similar results in both sexes (data not shown). Additional analyses using more typical PA survey cutoff values were also performed. The PA cutoff values were adapted from Paffenbarger et al. (27) and are as follows (with group sample sizes): 0–1000 (n = 18), 1001– 2000 (n = 13), 2001–3000 (n = 15), and 93000 (n = 23) kcalIwkj1 of EEE. Similar to the results shown in Figure 1, the 1001–2000 kcalIwkj1 group exhibited significantly longer telomere lengths than both the 0–1000 and the 93000 kcalIwkj1 groups (1.15 T 0.04 vs 0.95 T 0.04 and 0.97 T 0.03; P = 0.004) but was not significantly different from the 2001–3000 kcalIwkj1 group (1.02 T 0.04).
FIGURE 1.
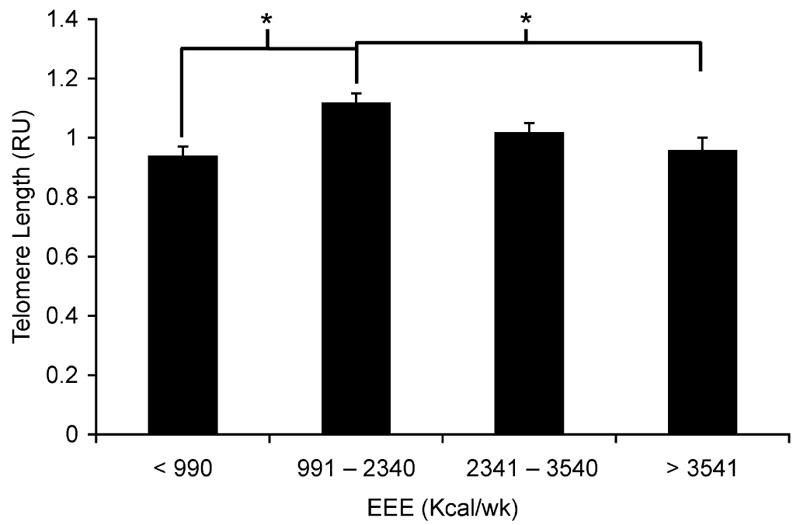
PBMC telomere length (relative telomere units, RU) is shown by EEE quartile (kcalIwkj1. *Significantly different from the second EEE quartile (P G 0.05). The second EEE quartile exhibited significantly longer telomere lengths (1.12 T 0.03 RU) than both the first and fourth EEE quartiles (0.94 T 0.03 and 0.96 T 0.03 RU, respectively; P G 0.05) but was not different from the third quartile.
Telomerase activity
ANCOVA revealed no differences among EEE groups for telomerase activity analyzed using either log-transformed data (not shown) or un-transformed data (Table 2).
TABLE 2.
Telomerase activity overall and by EEE quartile.
| N (F, M) |
||||||
|---|---|---|---|---|---|---|
| Overall (35, 34) | First (11, 6) | Second (12, 5) | Third (7, 8) | Fourth (5, 15) | P | |
| Telomerase activity (amol) |
3.67 • 10j3(1.7 • 10j2) | 1.35 • 10j3 T 4.2 • 10j3 | 1.68 • 10j3 T 4.2 • 10j3 | 2.26 • 10j3T 4.4 • 10j3 | 8.39 • 10j3 T 3.4 • 10j3 | 0.84 |
Values in the second column are reported as means (SD); all other values are reported as least square means T SE. Telomerase activity is measured in total product generated (TPG) per 10,000 cells; amol = attomoles.
hTERT genotype
Subject characteristics by hTERT genotype group are shown in Table 3. Across genotypes, subjects were statistically similar with respect to all variables except telomerase enzyme activity. The TT genotype had the greatest telomerase activity and was significantly different from both the CT and CC genotype groups (P = 0.01), as shown in Figure 2.
TABLE 3.
Subject characteristics, telomere length, and telomerase activity by hTERT genotype.
| CC | CT | TT | P | |
|---|---|---|---|---|
| N | 20 | 30 | 19 | - |
| Age (yr) | 59.9 T 1.14 | 60.9 T 0.91 | 60.2 T 1.11 | 0.75 |
| BMI (kgImj2) | 26.0 T 1.08 | 25.5 T 0.85 | 27.5 T 1.03 | 0.35 |
| PSS | 10.7 T 1.37 | 10.3 T 0.96 | 9.8 T 1.17 | 0.78 |
| EEE (kcalIwkj1) | 2435 T 532 | 3078 T 423 | 2376 T 518 | 0.49 |
| Telomere length (relative units) | 1.01 T 0.04 | 1.00 T 0.03 | 1.01 T 0.04 | 0.95 |
| Telomerase enzyme activity (TPG in amol) | 5.0 • 10j4 T 3.9 • 10j3 | 1.3 • 10j3 T 3.2 • 10j3 | 1.0 • 10j2 T 4.0 • 10j3* | 0.013a |
| Total EE (kcalIwkj1) | 9148 T 969 | 7880 T 771 | 7415 T 944 | 0.41 |
| Yale Summary | 63.2 T 5.6 | 66.5 T 4.4 | 55.4 T 5.4 | 0.29 |
| Yale Vigorous | 32.9 T 4.8 | 32.8 T 3.78 | 28.5 T 4.6 | 0.73 |
Data are means T SE. TPG, total product generated per 10,000 cells; amol = attomoles.
P value for the transformed data was used.
CC and CT genotypes groups were significantly different from TT (P G 0.05).
FIGURE 2.
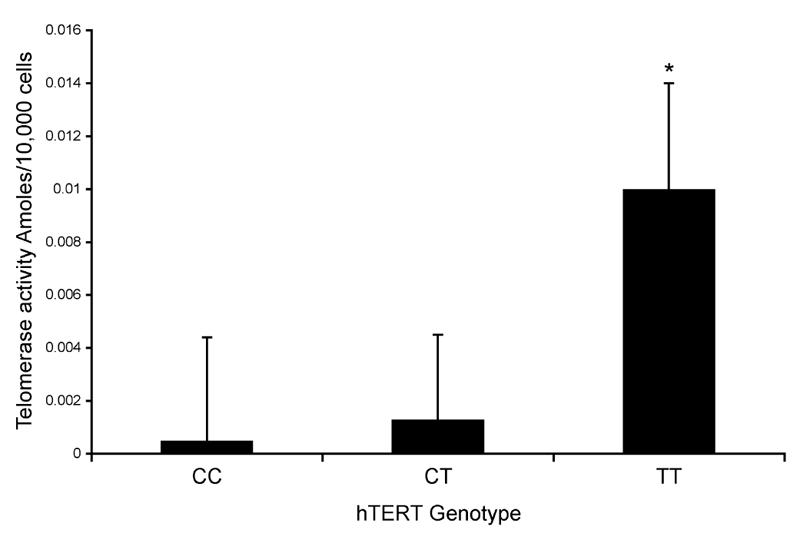
PBMC telomerase enzyme activity (total product generated per 10,000 cells) is shown by hTERT (TERT gene) C-1327T promoter genotype (CC, CT, and TT groups). *Significantly different telomerase enzyme activity (P G 0.05). The TT genotype (1.0 • 10j2 T 4.0 • 10j3 amoll 10,000 cellsj1; n = 19) exhibits significantly greater telomerase enzyme activity than both the CT (1.3 • 10j3 T 3.2 • 10j3; n = 30) and CC genotype groups (5.0 • 10j4 T 3.9 • 10j3; n = 20; P = 0.01).
Telomere length was not significantly different among genotype groups (P = 0.8; Table 3). When the interaction between genotype and EEE was added to the ANCOVA models, a significant interaction was observed for telomerase enzyme activity (P = 0.02; Table 4), such that the TT genotype/fourth EEE quartile individuals had significantly greater telomerase activity than all other groups. When Tukey’s HSD was used to control for comparison-wise error rate, the TT genotype/fourth EEE quartile individuals still had significantly greater telomerase activity than both the CC and CT genotype/fourth EEE quartile individuals and also the CC genotype/third EEE quartile individuals, as shown in Table 4. No such interaction was observed for telomere length (data not shown; P = 0.7).
TABLE 4.
Interaction between EEE and hTERT genotype with telomerase activity.
| hTERT Genotype |
|||
|---|---|---|---|
| EEE Quartile | CC | CT | TT |
| First | 1.3 • 10j3 T 7.5 • 10j3 | 1.5 • 10j3 T 6.4 • 10j3 | 1.2 • 10j3 T 7.5 • 10j3 |
| Second | 4.1 • 10j4 T 8.4 • 10j3 | 3.4 • 10j3 T 8.4 • 10j3 | 1.2 • 10j3 T 6.1 • 10j3 |
| Third | 3.0 • 10j4 T 6.9 • 10j3* | 4.2 • 10j3 T 6.0 • 10j3 | 6.7 • 10j4 T 1.8 • 10j2 |
| Fourth | 3.5 • 10j4 T 8.4 • 10j3* | 8.0 • 10j4 T 5.1 • 10j3* | 3.2 • 10j2 T 7.6 • 10j3 |
Data are LS means T SE for the interaction of EEE, genotype, and telomerase enzyme activity (TPG = amolI10,000 cellsj1 ). The overall interaction between EEE group and hTERT genotype was significant (P = 0.0195).
Groups were significantly different from high (fourth quartile) + TT genotype (P = 0.05 to P G 0.001).
Perceived stress survey
The overall and quartile group means for the PSS are shown in Table 1, with no significant differences observed among or between EEE quartiles (P = 0.12). When PSS was considered as a potential covariate for the analysis of EEE quartiles, PSS was not significantly associated with either telomere length or telomerase activity (P 9 0.2 for both; data not shown). We also examined whether PSS was associated with telomere length independent of EEE quartile. When subjects were grouped by PSS quartiles, PSS was not significantly associated with telomere length (P = 0.8; data not shown). Similarly, when subjects were grouped as upper and lower median splits of PSS values, the telomere length of the upper half (PSS 9 15) was not significantly different from the lower half (PSS G 8; P = 0.56, data not shown).
DISCUSSION
The present study reports a significant relationship between PA level and telomere length, such that moderate levels of PA are associated with a significantly longer PBMC telomere length compared with both the lowest and highest quartiles of PA. The breadth of PA levels included in the present study is larger than what has previously been examined. Our results for moderate PA levels are consistent with previous findings showing a positive dose—response relationship between leisure time PA and telomere length (11). No relationship was observed between PA level and telomerase enzyme activity in PBMC, contrary to our hypothesis. To our knowledge, no studies to date have directly investigated the relationship between PA level and telomerase enzyme activity. Finally, we observed a significant association between hTERT TT genotype and telomerase enzyme activity and a preliminary evidence for an interaction between hTERT genotype and PA level that deserves further exploration.
In the present study, PA level over the past 5 yr or longer was significantly associated with telomere length. Acute exercise or short-term PA alone may not be a strong enough stimulus to cause short-term changes to telomere length (29), but a protective effect may be manifested for 5 yr or more of consistent PA. The observed difference of 0.18 units between the first and second quartiles (and 0.2 units between the 0–1001 and the 1001–2000 kcalIwkj1 groups) is similar to differences (0.2 units) previously related to increased psychological stress (16) and to morbidity and poor survival rate from infectious and CVD (9); thus, the difference seems meaningful. Both the present study and the investigation by Cherkas et al. (11) used self-report PA as the main independent variable and took into consideration history of PA, although our study examined a greater range of PA levels. The present study included individuals who were competitive Master’s athletes and Senior Olympians, who reported greater than 6000 kcalIwkj1 of EEE, or who had a PA participation 5–7 dIwkj1 averaging 2 hIdj1. In the study by Cherkas et al. (11), the subjects in the heavy activity group reported only 9199 minIwkj1 of activity during their 20s, whereas the older subjects in our highest activity quartile were exercising 10–14 hIwkj1. In the present study, this highly active group exhibited telomere lengths no different from the lowest activity quartile, reflecting an “inverted U” curve relationship between increasing PA levels and telomere length, which contrasts with the linear dose—response relationship reported by Cherkas et al. (11). The difference in the two studies is likely due to the increased breadth of PA levels in the present study, allowing a broader view of the association.
Our findings, showing an association between EEE and PBMC telomere length, are congruent with the current literature on exercise training and immune function. A moderate level of endurance exercise training has been shown to elicit an enhanced immune response and reduced infection risk compared with both low (e.g., sedentary) and high (e.g., overtraining) exercise training levels (23,24), or an inverted U curve phenomenon. The telomere length data from the present study indicate that a similar relationship may be evident with EEE, with either low or high levels of PA associated with shorter telomere length compared with moderate levels of PA. PBMC are immune cells that are influenced by both acute exercise and regular exercise training (25,29). Acute endurance exercise of varying intensities is known to elicit a proliferative and functional response to the different subsets of PBMC including T and B lymphocytes and natural killer cells (29). Whether this highly active group experienced an increased need for immune cells after long-duration exercise events (24) or increased oxidative stress or associated antioxidant washout (3) is uncertain, but the present findings and those of Cherkas et al. (11) argue for future mechanistic studies in this area of telomere biology.
A study completed by Collins et al. (13) addressed similar questions, although their work was related to athletic overtraining rather than to more typical PA. They observed that muscle cell homogenate telomere length from highly trained endurance athletes was significantly longer compared with age- and training-matched athletes with FAMS (13). Some of our higher EEE subjects (i.e., fourth quartile) could have experienced “overtraining” in their lifetime and hence lead to shorter PBMC telomere length, although none of the subjects reported unusual fatigue or any acute myopathy as part of the medical screening. The findings of Collins et al. (13), Cherkas et al. (11), and the present study all provide support for the hypothesis that PA can modify telomere length, although the present findings and those of Collins et al. (13) provide evidence that very high PA levels may be detrimental to telomere length, as indicated by the inverted U relationship shown in Figure 1.
In contrast to telomere length, the present study did not observe a significant association between telomerase activity and PA levels. The relationship between telomerase enzyme activity and PA deserves further investigation. In an animal study of strenuous exercise training and telomerase enzyme activity in skeletal muscle, it was observed that mild or strenuous exercise training in rats did not significantly change telomerase enzyme activity (28). Although various environmental factors, such as oxidative stress, are known to up- and down-regulate telomerase activity (28), existing data indicate that exercise may not be such a stimulus. Future mechanistic studies are needed to address how PA may affect telomere length in those cells with telomerase enzyme activity (e.g., PBMC).
Matsubara et al. (23) showed that individuals with the TT genotype of a promoter SNP in the TERT gene had greater hTERT promoter activity, significantly longer telomeres, and greater telomerase enzyme activity in leukocytes than individuals with the CC genotype. In a second case—control study, Matsubara et al. (22) observed a significant relationship between the CC genotype and coronary artery disease. Our findings partially support those of Matsubara et al. (23) in that we observed greater telomerase enzyme activity in the TT genotype group compared with both CT and CC groups. We did not observe a relationship between TT genotype and telomere length in the present study, consistent with recent findings of Nordfjall et al. (26) who also reported that telomere length was not related to the hTERT genotype. Of interest in the present study was the finding of a significant interaction between quartiles of EEE and hTERT genotype in relation to telomerase activity. This finding should be considered preliminary given the small sample sizes, but the results warrant further exploration.
The present study is not without limitations. The data are observational in nature, so mechanistic underpinnings can only be speculated. The PA information obtained was from self-report questionnaire. Although the YPAS questionnaire has been widely used and validated (15,31), under- and overreporting of PA occurs in studies of this nature, and recall bias may have occurred. Future studies should consider more sophisticated methods to assess PA. Another limitation was that oxidative stress was not directly measured or statistically controlled; hence, possible underlying mechanisms of the observed associations cannot be addressed in the current study. Oxidative stress is known to affect telomere biology, and vitamin supplementation, specifically antioxidant intake, was not controlled for and could have confounded the results. The goal of the present study was to test the basic hypothesis that PA level is related to telomere biology, which was supported and thus provided a foundation for future, mechanistic studies. Our study was strengthened, however, by using similarly aged, healthy individuals with low levels of psychological stress and similar SES measures, all factors which could have confounded our results had they not been homogeneous. The narrow age span (20 yr) used purposefully in the present study was used because our goal was not to show the affect of age on telomere length per se but rather the relationship between lifetime PA and telomere length. Finally, no significant findings were observed for psychological stress in the present study. The PSS used in the present study indicated relatively low stress levels across EEE quartiles, which prevented a direct replication of previous work in this area. Moreover, our PSS examined recent rather than lifelong stress levels; future studies should consider the possibility of an interaction between lifelong EEE and psychological stress in relation to telomere length and telomerase activity.
In summary, we extend previous findings by confirming that moderate PA may be beneficial to telomere length in PBMC but show that higher PA levels may not have such a positive relationship. A recent study examining long-term strength training did not observe a benefit for skeletal muscle telomere length (20). We did not observe an association between PA levels and telomerase activity, although we did confirm results from two previous studies of an association between hTERT genotype and telomerase enzyme activity. Also, an intriguing genotype • PA interaction was observed, providing hypotheses for future investigations. Longitudinal studies looking at PA are necessary to both confirm and gain insight into the mechanisms underlying the present findings, which provide evidence that long-term PA patterns may directly alter cellular aging.
Acknowledgments
The authors thank the subjects who made this investigation possible. Jade Clark assisted with the telomere length and telomerase assays. This work was sponsored by grants AG025505 and AG022791 from the National Institutes of Health. The results of the present study do not constitute endorsement by ACSM.
REFERENCES
- 1.Benetos A, Gardner JP, Zureik M, et al. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43(2):182–5. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 2.Benetos A, Okuda K, Lajemi M, et al. Telomere length as an indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37(2):381–5. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 3.Bergholm R, Makimattila S, Valkonen M, et al. Intense physical training decreases circulating antioxidants and endothelium-dependent vasodilatation in vivo. Atherosclerosis. 1999;145(2):341–9. doi: 10.1016/s0021-9150(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn EH. Telomere states and cell fates. Nature. 2000;408(6808):53–6. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn EH. Telomeres and telomerase. Keio J Med. 2000;49(2):59–65. doi: 10.2302/kjm.49.59. [DOI] [PubMed] [Google Scholar]
- 6.Blair SN, Kohl HW, III, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273(14):1093–8. [PubMed] [Google Scholar]
- 7.Carter CS, Hofer T, Seo AY, Leeuwenburgh C. Molecular mechanisms of life- and health-span extension: role of calorie restriction and exercise intervention. Appl Physiol Nutr Metab. 2007;32:954–66. doi: 10.1139/H07-085. [DOI] [PubMed] [Google Scholar]
- 8.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 10.Cherkas LF, Aviv A, Valdes AM, et al. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5(5):361–5. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 11.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168(2):154–8. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–96. [PubMed] [Google Scholar]
- 13.Collins M, Renault V, Grobler LA, et al. Athletes with exercise-associated fatigue have abnormally short muscle DNA telomeres. Med Sci Sports Exerc. 2003;35(9):1524–8. doi: 10.1249/01.MSS.0000084522.14168.49. [DOI] [PubMed] [Google Scholar]
- 14.Demissie S, Levy D, Benjamin EJ, et al. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325–30. doi: 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 15.Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc. 1993;25(5):628–42. [PubMed] [Google Scholar]
- 16.Epel ES, Blackburn EH, Lin J, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–5. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epel ES, Lin J, Wilhelm FH, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–87. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 18.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res. 1965;37:614–36. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- 19.Hiyama K, Hirai Y, Kyoizumi S, et al. Activation of telomerase in human lymphocytes and hematopoietic progenitor cells. J Immunol. 1995;155(8):3711–5. [PubMed] [Google Scholar]
- 20.Kadi F, Ponsot E, Piehl-Aulin K, et al. The effects of regular strength training on telomere length in human skeletal muscle. Med Sci Sports Exerc. 2008;40:82–7. doi: 10.1249/mss.0b013e3181596695. [DOI] [PubMed] [Google Scholar]
- 21.Lee I-M, Paffenbarger RS, Hennekens CH. Physical activity, physical fitness and longevity. Aging (Milano) 1997;9:2–11. doi: 10.1007/BF03340123. [DOI] [PubMed] [Google Scholar]
- 22.Matsubara Y, Murata M, Watanabe K, et al. Coronary artery disease and a functional polymorphism of hTERT. Biochem Biophys Res Commun. 2006;348(2):669–72. doi: 10.1016/j.bbrc.2006.07.103. [DOI] [PubMed] [Google Scholar]
- 23.Matsubara Y, Murata M, Yoshida T, et al. Telomere length of normal leukocytes is affected by a functional polymorphism of hTERT. Biochem Biophys Res Commun. 2006;341(1):128–31. doi: 10.1016/j.bbrc.2005.12.163. [DOI] [PubMed] [Google Scholar]
- 24.Nieman DC. Immune response to heavy exertion. J Appl Physiol. 1997;82(5):1385–94. doi: 10.1152/jappl.1997.82.5.1385. [DOI] [PubMed] [Google Scholar]
- 25.Nieman DC. Is infection risk linked to exercise workload? Med Sci Sports Exerc. 2000;32(7 Suppl):S406–11. doi: 10.1097/00005768-200007001-00005. [DOI] [PubMed] [Google Scholar]
- 26.Nordfjall K, Osterman P, Melander O, Nilsson P, Roos G. hTERT (j 1327)T/C polymorphism is not associated with age-related telomere attrition in peripheral blood. Biochem Biophys Res Commun. 2007;358(1):215–8. doi: 10.1016/j.bbrc.2007.04.099. [DOI] [PubMed] [Google Scholar]
- 27.Paffenbarger RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med. 1986;314:605–13. doi: 10.1056/NEJM198603063141003. [DOI] [PubMed] [Google Scholar]
- 28.Radak Z, Taylor AW, Sasvari M, et al. Telomerase activity is not altered by regular strenuous exercise in skeletal muscle or by sarcoma in liver of rats. Redox Rep. 2001;6(2):99–103. doi: 10.1179/135100001101536102. [DOI] [PubMed] [Google Scholar]
- 29.Rowbottom DG, Green KJ. Acute exercise effects on the immune system. Med Sci Sports Exerc. 2000;32(7 Suppl):S396–405. doi: 10.1097/00005768-200007001-00004. [DOI] [PubMed] [Google Scholar]
- 30.Shin YA, Lee JH, Song W, Jun TW. Exercise training improves the antioxidant enzyme activity with no changes of telomere length. Mech Ageing Dev. 2008;129:254–60. doi: 10.1016/j.mad.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Young DR, Jee SH, Appel LJ. A comparison of the Yale Physical Activity Survey with other physical activity measures. Med Sci Sports Exerc. 2001;33(6):955–61. doi: 10.1097/00005768-200106000-00015. [DOI] [PubMed] [Google Scholar]


