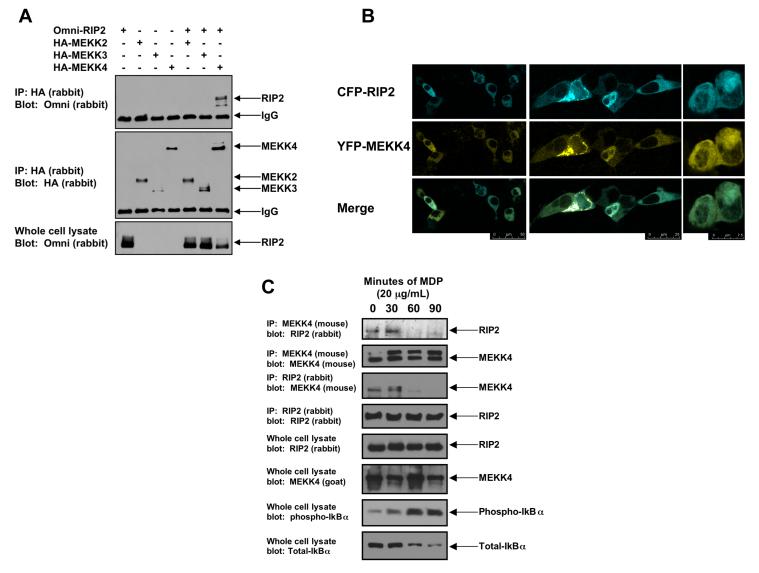
Figure 1. MEKK4 binds to endogenous RIP2, and NOD2 activation causes dissociation of the RIP2:MEKK4 complex.
A. In a screening strategy designed to identify the MAP3Ks that bind to RIP2, HA-tagged-MEKK2, MEKK3 and MEKK4 were co-transfected into 293 cells with Omni-tagged RIP2. After immunoprecipitation, Western blotting was performed using the indicated antibodies.
B. To determine if RIP2 and MEKK4 co-localized in the cell, CFP-RIP2 and YFP-MEKK4 fusion constructs were generated. 293 cells were transfected, and confocal microscopy was performed. In the basal, unstimulated state, RIP2 and MEKK4 co-localized. Multiple views with merging of the images are shown.
C. To determine if endogenous RIP2 and MEKK4 bound one another and if this complex persists in response to NOD2 activation (via MDP exposure), RAW 264.7 cells were stimulated with 20 μg/mL MDP for 0, 30, 60 or 90 minutes. Cells were lysed and subjected to immunoprecipitation with an anti-MEKK4 antibody or an anti-RIP2 antibody. Western blotting was performed after extensive washing of the immunoprecipitate. As a control for MDP activation, lysates were Western blotted using anti-phospho-IκBα and anti-total IκBα. Endogenous MEKK4 binds to endogenous RIP2 in the unstimulated state. As the NFκB pathway is activated (shown by the anti-phospho-IκBα blot), MEKK4 binding to RIP2 greatly decreases. This pattern is seen in reciprocal co-immunoprecipitations.