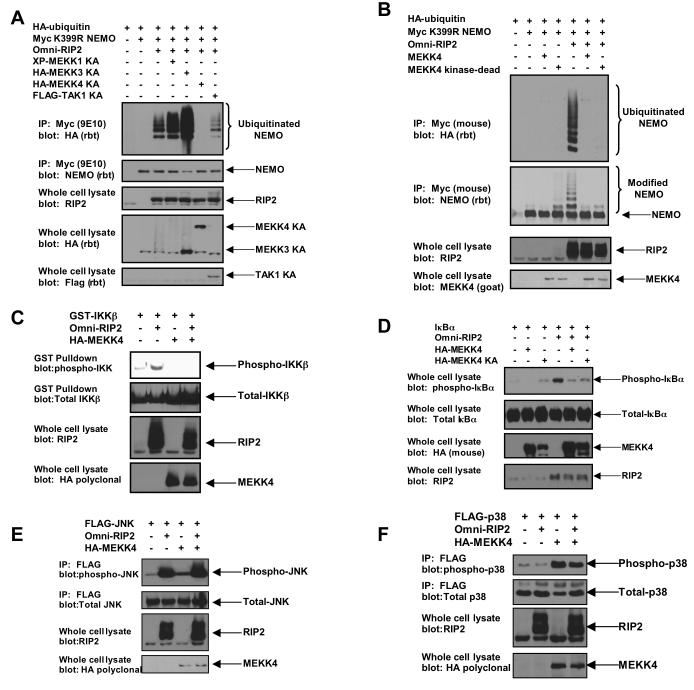
Figure 3. MEKK4 expression inhibits RIP2-induced NEMO ubiquitination and RIP2-induced IKK activation.
A. To determine the effect of MEKK4 on RIP2-induced NEMO ubiquitination and to determine the MAP3K specificity of this effect, kinase-dead (denoted as KA — catalytic lysine converted to alanine in each kinase) variants of MEKKs 1, 3 and 4 were transfected into 293s with myc-tagged K399R NEMO, RIP2 and HA-tagged ubiquitin. As a positive control for inhibition of NEMO ubiquitination, kinase dead TAK1 was utilized. After immunoprecipitating NEMO under stringent washing conditions, Western blotting was performed using the indicated antibodies. Kinase-dead MEKK4 specifically and strongly inhibited RIP2-induced NEMO ubiquitination.
B. To determine whether the inhibitory effect of MEKK4 on RIP2-induced NEMO ubiquitination was dependent on the kinase activity of MEKK4, wt MEKK4 or kinase-dead MEKK4 was transfected into 293 cells with myc-tagged K399R NEMO, RIP2 and HA-tagged ubiquitin. After immunoprecipitation, Western blotting was performed. wt MEKK4 inhibited RIP2-induced NEMO ubiquitination with similar efficacy as kinase-dead MEKK4.
C. Mammalian GST-tagged IKKβ was transfected into 293 cells with RIP2 and/or MEKK4. After purification with glutathione-sepharose beads, Western blots were performed. MEKK4 strongly inhibits RIP2-induced IKKβ activation.
D. 293 cells were transfected with IκBα, RIP2 and/or wt MEKK4 or kinase-dead (KA) MEKK4. Lysates were generated and Western blotting was performed. RIP2 strongly induces IκBα phosphorylation and both wt and kinase-dead (KA) MEKK4 inhibit this activation with similar efficacy.
E. Flag-tagged JNK was transfected into 293 cells with RIP2 and/or MEKK4. After immunoprecipitation with the Flag antibody, Western blots were performed with the indicated antibodies. RIP2 strongly activates JNK, and MEKK4 expression has no effect on this activation.
F. Flag-tagged p38 was transfected into 293 cells with RIP2 and/or MEKK4. After immunoprecipitation with the Flag antibody, Western blots were performed. MEKK4 strongly activates p38. RIP2 co-expression had little effect on this activation.