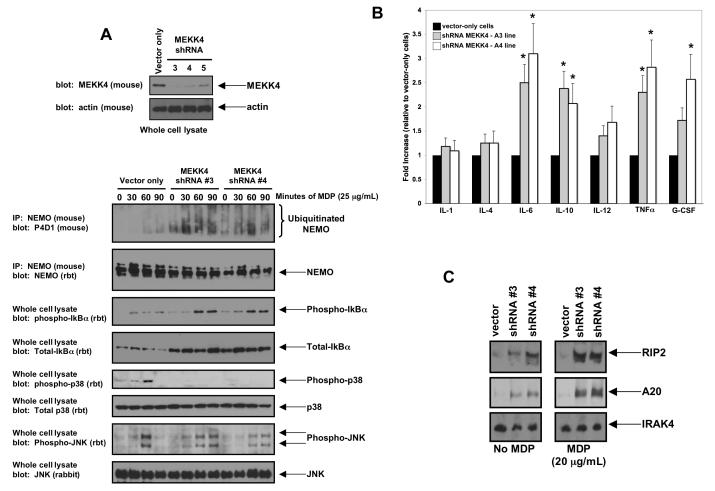
Figure 4. Decreased expression of MEKK4 causes increased MDP-induced NEMO ubiquitination, increased MDP-induced NFκB activation, decreased MDP-induced p38 activation and increased basal cytokine production.
A. RAW 264.7 cells were transduced with individual lentiviruses targeting MEKK4. As a control, an empty lentivirus was used. Western blotting was performed to determine the degree of inhibition of MEKK4 expression. Cell lines MEKK4 shRNA #3 and MEKK4 shRNA #4 showed the greatest loss of expression and were used for subsequent experiments. These cell lines were then exposed to MDP for 0, 30, 60 or 90 minutes. Lysates were generated, and half the lysates were subjected to immunoprecipitation stringent washing conditions (0.25% SDS, 1 M NaCl). Western blots showed that NEMO ubiquitination was increased basally and greatly increased under stimulation in the shRNA#3 and shRNA#4 cell lines but not in the vector only cell line. In agreement with this finding, phospho-IκBα was increased in these cell lines (both basally and with stimulation), indicating higher NFκB activity. In contrast, while JNK signaling was similar between the vector-only cell line and the shRNA#3 and the shRNA#4 cell lines, p38 signaling was greatly decreased with MEKK4 expression was inhibited..
B. To determine if this increased basal NFκB activation resulted in altered cytokine production, cytokine levels were assayed from these cell lines by ELISA. IL-6, IL-10, IL-12, TNFα and G-CSF were all significantly basally upregulated in both MEKK4 knockdown lines. Student’s t-test showed that IL-6, IL-10, TNF and G-CSF increases had a significance of P<0.05 (indicated by *). As these cytokines are all regulated by NFκB, these findings suggest a functional role for the increased NFκB signaling seen in Figure 4A.
C. Expression of two NFκB-regulated proteins was then assayed under either basal conditions or after overnight MDP stimulation. Both RIP2 and A20 were significantly upregulated in the MEKK4 knockdown lines. In contrast, the innate immunity protein, IRAK4, whose expression is not regulated by NFκB activity, was essentially unchanged.