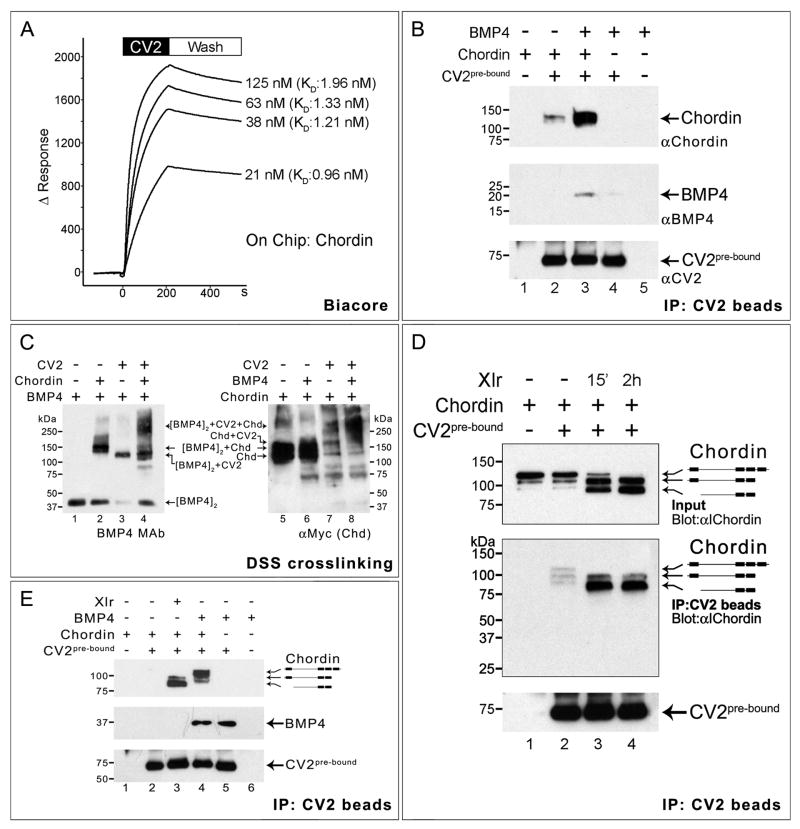
Figure 5. CV2 and Chordin Bind to Each Other.
(A) Biacore sensograms of the CV2-Chordin interaction in real-time showing an average KD of 1.37 nM. The time at which the binding of CV2 protein stops and the buffer wash starts is indicated.
(B) Co-immunoprecipitation in solution showing that Chordin and BMP4 bind better to CV2 in the presence of each other. Top panel shows Western blot immunostained with anti-Chordin, middle panel with monoclonal anti-BMP4, and bottom panel (loading control) with anti-CV2 antibody.
(C) CV2, Chordin and BMP4 form a ternary complex. Left panel shows crosslinked products stained with BMP4 monoclonal antibody; the antigenicity of BMP4 in crosslinked complexes with Chd and CV2 increases (the unbound BMP4 dimer band indicates the amount of BMP4 protein present in the complexes). The right panel the same products stained for myc-tagged Xenopus Chordin protein. The position of the BMP4/CV2/Chordin ternary complex is indicated.
(D) CV2 binds better to Chordin cleavage products than to Chordin full-length protein. Some cleavage products were present in the Chordin protein preparation, and these were enriched in lane 2.
(E) BMP4 pre-bound to Chordin enhances the binding preference of CV2 beads for full-length Chordin (lane 4). At this exposure level the binding of full-length Chordin to CV2 is undetectable (lane 2), but is greatly increased by adding Xlr or BMP4 (lanes 3 and 4).