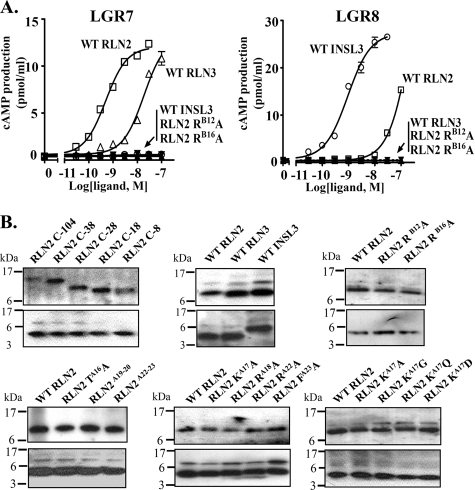
FIGURE 5.
Stimulation of cAMP production in LGR7- and LGR8-expressing cells by recombinant RLN2, RLN3, and INSL3. A, recombinant RLN3 and INSL3 selectively activated LGR7 and LGR8, respectively. In contrast, RLN2 activates both receptors at the nanomolar range. Unlike the wild type RLN2, substitution of ArgB12 and ArgB16 residues with alanine abolished the LGR7- and LGR8-activation activities of RLN2. Each data point represents the mean ± S.E. of quadruplicate samples. B, Western blotting analysis of recombinant wild type and mutant relaxin peptides including, RLN2 C-104, C-38, C-28, C-18, C-8 (upper left), WT RLN2, WT RLN3, WT INSL3 (upper middle), WT RLN2, RLN2 RB12A, RLN2 RB16A (upper right), WT RLN2, RLN2 TA16A, RLN2A19-20, RLN2A22-23 (lower left), WT RLN2, RLN2 KA17A, RLN2 RA18A, RLN2 RA22A, RLN2 FA23A (lower middle), WT RLN2, RLN2 KA17A, RLN2 KA17G, RLN2 KA17Q, and RLN2 KA17D(lower right). Affinity column purified peptides (100 ng/lane) were resolved by 18% SDS-PAGE under nonreducing (upper panel) and reducing (lower panel) conditions. The positions of molecular mass markers are indicated on the left.