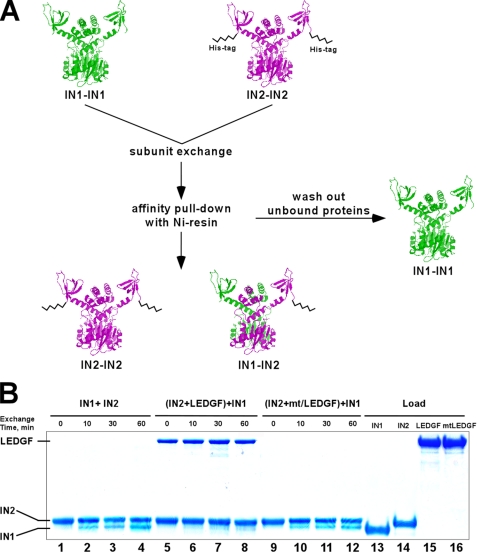
FIGURE 3.
Subunit exchange assay using His-tag IN and SDS-PAGE: design (A) and results (B). A, subunit exchange between IN multimers was tested by mixing two different IN proteins: IN1, a tag-free form and IN2, containing the 6× His tag at its C terminus. The full-length proteins are depicted as dimers (IN1-IN1) and (IN2-IN2). Both proteins contained wild-type IN sequences and displayed identical catalytic activities. After mixing, unbound proteins were washed from the resin and bound complexes were analyzed by SDS-PAGE. IN1 and IN2 were clearly separated based on their molecular weight differences. B, data in lanes 1–4 indicate that IN2 was able to quantitatively pull-down tag-free IN1. The recovered multimers contained a mixture of IN2-IN2 and IN1-IN2, while tag-free IN1-IN1 was washed out from the Ni-NTA resin. To assess the impact of LEDGF on IN subunit exchange, the IN2 multimer was preincubated with LEDGF and then exposed to IN1 (lanes 5–8). The results show that LEDGF interacted with IN2 and effectively prevented IN subunit exchange (lanes 5–8). In contrast, mtLEDGF did not bind IN2 or affect subunit exchange (lanes 9–12). Total amounts of input IN1, IN2, LEDGF, and mtLEDGF proteins are shown in lanes 13, 14, 15, and 16, respectively.