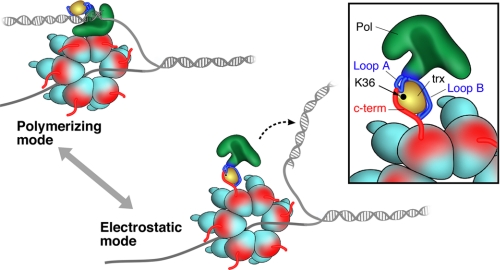
FIGURE 7.
Two modes of binding of gp5/trx with gp4. In the polymerizing mode, gp5-trx and gp4 form a tight complex that is independent of the C-terminal tail of gp4. The complex synthesizes an average of 5 kb of DNA before gp5/trx dissociates from the DNA. In the electrostatic mode, gp5/trx forms an unstable complex with gp4 via the C-terminal tail. This latter mode provides a mechanism for the exchange of the DNA polymerase at the replication fork without affecting the processivity. The inset displays the acidic C-terminal tail of gp4 electrostatically interacting with the basic residues in loop A and B of the TBD and lysine 36 of trx.