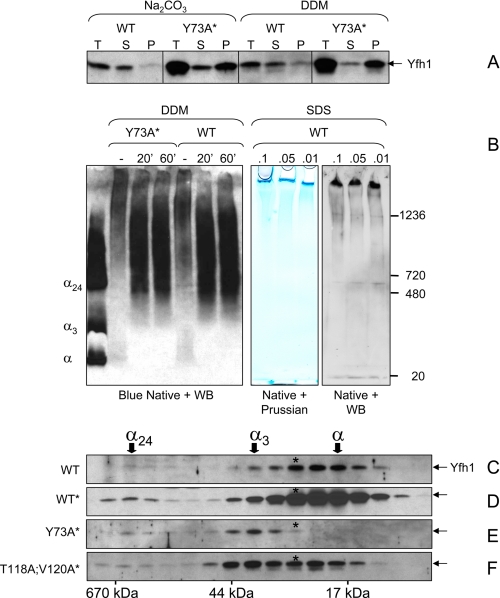
FIGURE 6.
Polymerization of Yfh1 in response to heat stress. A, mitochondria were isolated under the same conditions used in Fig. 5 (A–C), and the total mitochondrial lysates (T) were extracted with 100 mm Na2CO3 or 1% dodecyl maltoside for 30 min on ice prior to separation into supernatant (S) and pellet (P). B, total mitochondrial lysates (4.5 mg of total protein/ml) were left untreated or extracted with 1% dodecyl maltoside for 20 or 60 min on ice. After centrifugation at 10,000 × g for 20 min at 4 °C, aliquots of the supernatants were analyzed by blue native PAGE and Western blotting (WB) with anti-Yfh1 antibody. Purified monomer, trimer, and 24-mer (α, α3, and α24) were used as molecular mass standards. In another experiment, total mitochondrial lysates were incubated in the presence of low concentrations of SDS followed by centrifugation at 5,000 × g for 20 min. Two identical sets of samples from the resulting supernatants were analyzed by native PAGE (3–12% Bis-Tris) on the same gel. One half of the gel was stained with Prussian blue for iron detection, and the second half was analyzed by Western blotting for Yfh1 detection. C and F, yeast cells were preconditioned in SD and further grown in 1,500 ml of SD in the presence of 100μm FeCl3 for ∼24 h at 37 °C. The mitochondria were isolated, and a soluble mitochondrial fraction (1.5 mg of total protein) was analyzed by size exclusion chromatography and Western blotting as described in the legend of Fig. 3 (C–F). Because of the very low levels of soluble Yfh1 present in mitochondria under the conditions used in these experiments, long exposures were required for Yfh1 detection resulting in high backgrounds.