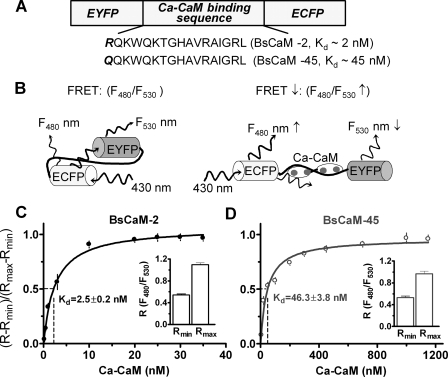
FIGURE 1.
FRET-based Ca2+-CaM biosensor. A, the domain structure of Ca2+-CaM biosensor. Two versions of the biosensor were developed: BsCaM-2 with a high Ca2+-CaM binding affinity versus BsCaM-45 with a lower Ca2+-CaM binding affinity similar to that of CaMKII. B, diagram showing the Ca2+-CaM-dependent FRET changes of the biosensors. C and D, titration of BsCaM-2 (C) and BsCaM-45 (D) in the presence of 50 μm Ca2+ with increasing concentrations of CaM. The data were fitted to a standard single-site binding model. The Kd values were obtained as 2.5 nm (BsCaM-2) and 46.3 nm (BsCaM-45), respectively. R is the ratio of F480 to F530. Rmin (the minimal ratio of biosensor in absence of Ca2+-CaM) and Rmax (the maximal ratio of biosensor saturated with Ca2+-CaM) are shown in the insets.