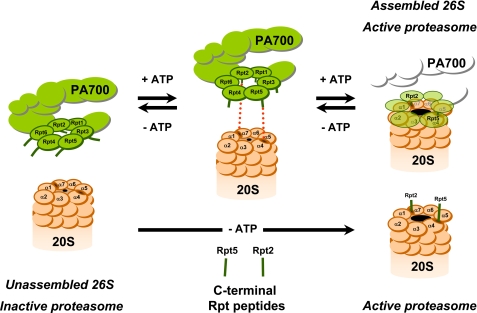
FIGURE 6.
Model for PA700 binding to and activation of the proteasome via COOH termini of Rpt2 and Rpt5 subunits. ATP binding to Rpt subunits of PA700 promotes conformational changes that optimize registration of COOH termini of Rpt2 and Rpt5 for interaction with cognate binding sites at proteasome subunits α7 and α4, respectively. Binding of these subunits induces conformational changes in α subunits of proteasome, resulting in gate opening and an increase in substrate access to catalytic sites. Peptides corresponding to the COOH termini of Rpt2 and/or Rpt5 can bind directly to their respective sites on α subunits of the proteasome to promote gate opening and proteasome activation by the same mechanism. Because no conformational change of PA700 is required for peptide binding, proteasome activation is ATP-independent. The nomenclature for 20 S proteasome subunits corresponds to that of Groll et al. (9).