Abstract
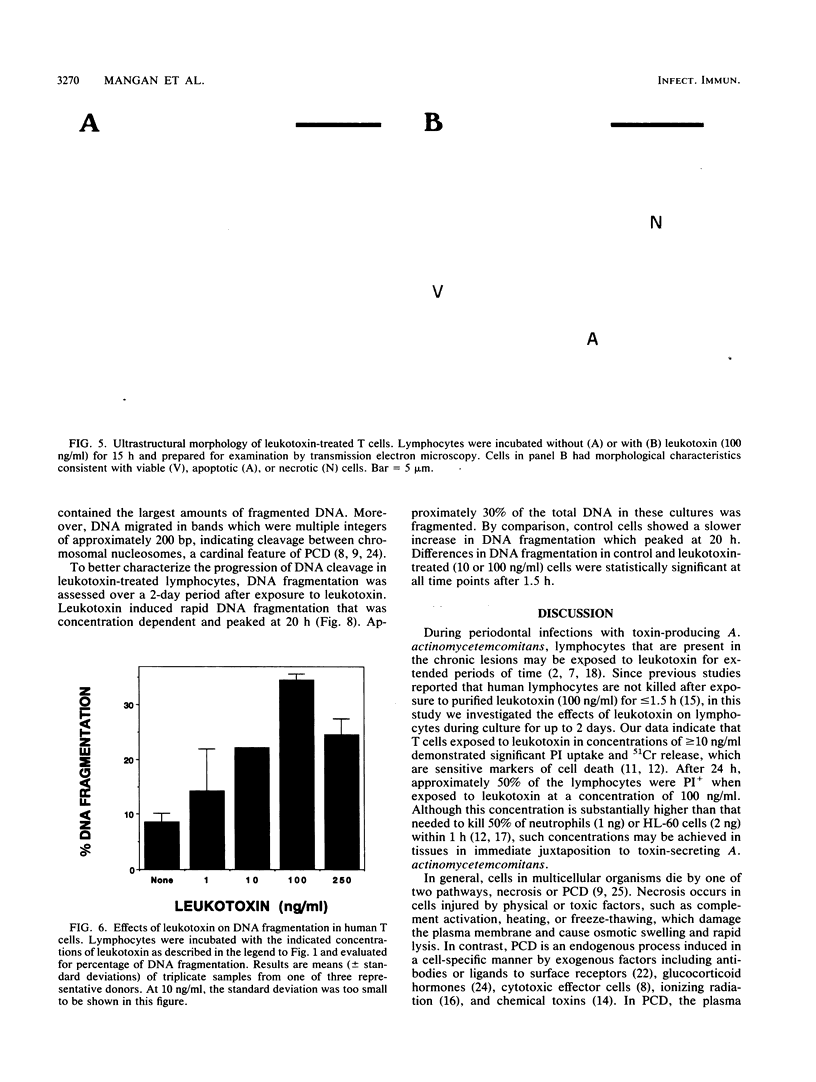
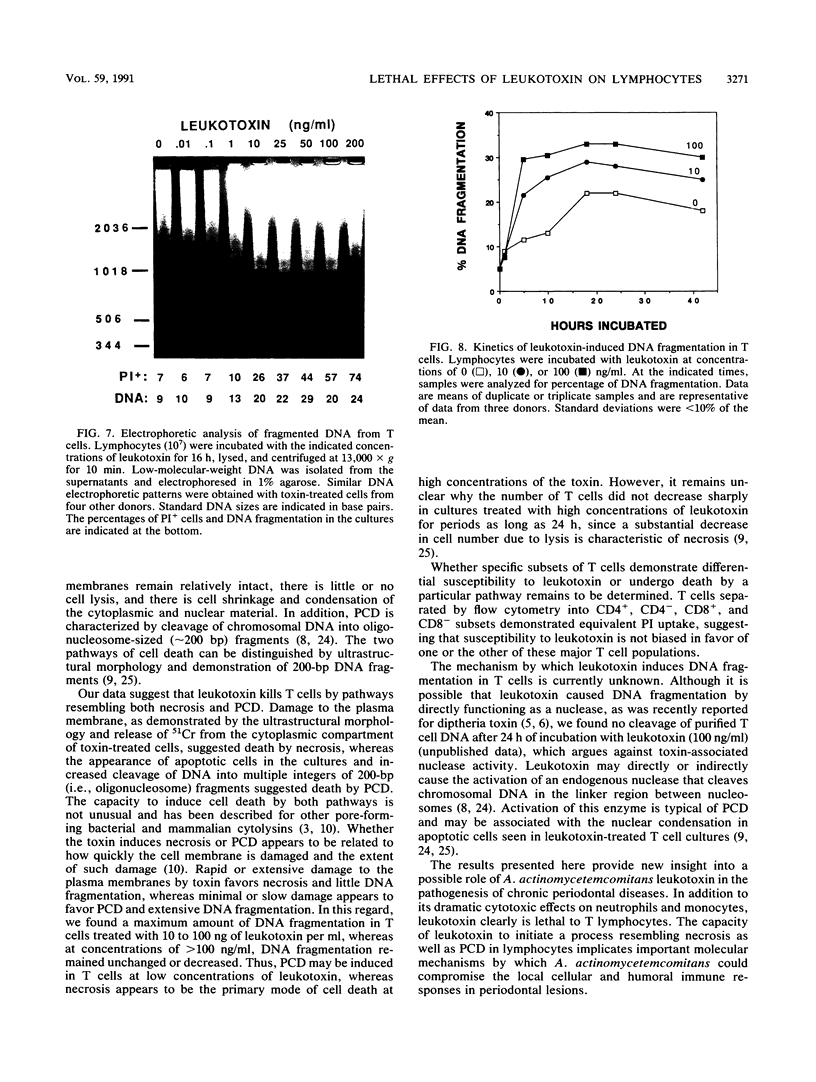
The majority of strains of Actinobacillus actinomycetemcomitans isolated from patients with periodontal diseases secrete a leukotoxin that destroys human myeloid cells within minutes but has no effect on viability of peripheral blood lymphocytes in culture for 1.5 h. However, since this organism persists in the gingival crevice and thus may continuously release toxin over extended periods of time, we assessed the viability of T cells cultured with leukotoxin (0 to 250 ng/ml) for up to 2 days. Although the total numbers of cells recovered from cultures with or without leukotoxin were equivalent, leukotoxin killed up to 70% of the T cells in a time- and concentration-dependent manner. Cell death was associated with uptake of propidium iodide, release of 51Cr from the cytoplasm, and morphological evidence of damage to the plasma membrane and apoptosis. Leukotoxin also induced increased cleavage of chromosomal DNA into nucleosome-sized fragments, suggesting activation of an endogenous nuclease in the T cells. These data suggest that leukotoxin kills T cells by pathways resembling necrosis and programmed cell death. Leukotoxin-induced lymphotoxicity may represent a critical mechanism by which A. actinomycetemcomitans suppresses the host local immune response and contributes to the pathogenesis of diseases involving this microorganisms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baehni P. C., Tsai C. C., McArthur W. P., Hammond B. F., Shenker B. J., Taichman N. S. Leukotoxic activity in different strains of the bacterium Actinobacillus actinomycetemcomitans isolated from juvenile periodontitis in man. Arch Oral Biol. 1981;26(8):671–676. doi: 10.1016/0003-9969(81)90164-3. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to cell membranes by pore-forming bacterial cytolysins. Prog Allergy. 1988;40:1–43. [PubMed] [Google Scholar]
- Chang M. P., Baldwin R. L., Bruce C., Wisnieski B. J. Second cytotoxic pathway of diphtheria toxin suggested by nuclease activity. Science. 1989 Dec 1;246(4934):1165–1168. doi: 10.1126/science.2531465. [DOI] [PubMed] [Google Scholar]
- Chang M. P., Bramhall J., Graves S., Bonavida B., Wisnieski B. J. Internucleosomal DNA cleavage precedes diphtheria toxin-induced cytolysis. Evidence that cell lysis is not a simple consequence of translation inhibition. J Biol Chem. 1989 Sep 15;264(26):15261–15267. [PubMed] [Google Scholar]
- Christersson L. A., Albini B., Zambon J. J., Wikesjö U. M., Genco R. J. Tissue localization of Actinobacillus actinomycetemcomitans in human periodontitis. I. Light, immunofluorescence and electron microscopic studies. J Periodontol. 1987 Aug;58(8):529–539. doi: 10.1902/jop.1987.58.8.529. [DOI] [PubMed] [Google Scholar]
- Duke R. C., Chervenak R., Cohen J. J. Endogenous endonuclease-induced DNA fragmentation: an early event in cell-mediated cytolysis. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6361–6365. doi: 10.1073/pnas.80.20.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A., Olsen K. J., Lee M. K., Lichtenheld M. G., Podack E. R. Cytolysis by Ca-permeable transmembrane channels. Pore formation causes extensive DNA degradation and cell lysis. J Exp Med. 1989 Mar 1;169(3):765–777. doi: 10.1084/jem.169.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase M., Lally E. T., Berthold P., Korchak H. M., Taichman N. S. Effects of cations and osmotic protectants on cytolytic activity of Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 1990 Jun;58(6):1782–1788. doi: 10.1128/iai.58.6.1782-1788.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan D. F., Welch G. R., Wahl S. M. Lipopolysaccharide, tumor necrosis factor-alpha, and IL-1 beta prevent programmed cell death (apoptosis) in human peripheral blood monocytes. J Immunol. 1991 Mar 1;146(5):1541–1546. [PubMed] [Google Scholar]
- Pritchard D. J., Butler W. H. Apoptosis--the mechanism of cell death in dimethylnitrosamine-induced hepatotoxicity. J Pathol. 1989 Jul;158(3):253–260. doi: 10.1002/path.1711580314. [DOI] [PubMed] [Google Scholar]
- Rabie G., Lally E. T., Shenker B. J. Immunosuppressive properties of Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 1988 Jan;56(1):122–127. doi: 10.1128/iai.56.1.122-127.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987 Nov 15;139(10):3199–3206. [PubMed] [Google Scholar]
- Simpson D. L., Berthold P., Taichman N. S. Killing of human myelomonocytic leukemia and lymphocytic cell lines by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun. 1988 May;56(5):1162–1166. doi: 10.1128/iai.56.5.1162-1166.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Listgarten M. A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988 Feb;15(2):85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Taichman N. S., Dean R. T., Sanderson C. J. Biochemical and morphological characterization of the killing of human monocytes by a leukotoxin derived from Actinobacillus actinomycetemcomitans. Infect Immun. 1980 Apr;28(1):258–268. doi: 10.1128/iai.28.1.258-268.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., McArthur W. P., Baehni P. C., Hammond B. F., Taichman N. S. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect Immun. 1979 Jul;25(1):427–439. doi: 10.1128/iai.25.1.427-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. C., Shenker B. J., DiRienzo J. M., Malamud D., Taichman N. S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect Immun. 1984 Feb;43(2):700–705. doi: 10.1128/iai.43.2.700-705.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucker D. S., Ashwell J. D., Nickas G. Activation-driven T cell death. I. Requirements for de novo transcription and translation and association with genome fragmentation. J Immunol. 1989 Dec 1;143(11):3461–3469. [PubMed] [Google Scholar]
- Wahl L. M., Katona I. M., Wilder R. L., Winter C. C., Haraoui B., Scher I., Wahl S. M. Isolation of human mononuclear cell subsets by counterflow centrifugal elutriation (CCE). I. Characterization of B-lymphocyte-, T-lymphocyte-, and monocyte-enriched fractions by flow cytometric analysis. Cell Immunol. 1984 May;85(2):373–383. doi: 10.1016/0008-8749(84)90251-x. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]