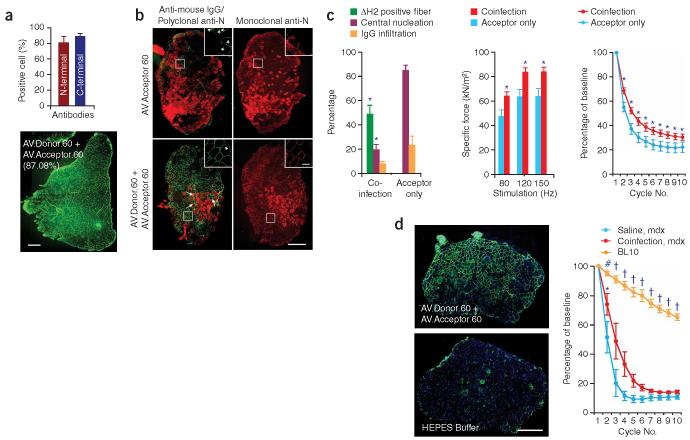
Figure 3.
Widespread expression of mini-dystrophin in AV.Donor.60 and AV.Acceptor.60 co-infected mdx muscle attenuates dystrophic pathology. (a) Mini-dystrophin expression in adult mdx TA muscle. 2 × 1010 vg particles of each virus were co-delivered to the TA muscles of 2-month-old male mdx mice. Expression was determined 3 months later by immunostaining. Bar graph represents the transduction efficiency quantified according to immunostaining with monoclonal antibodies. n = 6 for N-terminal antibody and n = 5 for C-terminal antibody. A representative montage composite photomicrograph from an entire TA muscle is shown in the bottom panel. The dystrophin-positive myofibers were revealed by a polyclonal N-terminal antibody. Number in parenthesis indicates the percentage of dystrophin-positive myofibers in the section. Scale bar, 500 μm. (b) Mini-dystrophin expression in adult EDL muscle prevents sarcolemma leakage. Equal amounts (5 × 109 vg particles) of each virus were co-injected into the left EDL muscles of 2-month-old male mdx mice. 5 × 109 vg particles of AV.Acceptor.60 was injected to the contralateral EDL muscles. Mini-dystrophin expression and sarcolemma integrity were examined 3 months later. Photomicrographs are from the representative EDL muscles. Sarcolemma leakage in injured myofibers is revealed by the uptake of immunoglobulin from circulation (anti-mouse lgG staining). Dystrophin expression is detected either by a polyclonal anti-N-terminal antibody (for both mini-dystrophin and revertant dystrophin) or a monoclonal anti-N-terminal antibody (only detects human dystrophin–derived mini-dystrophin). The overlay image of anti-mouse lgG staining (red) and polyclonal antibody immunostaining (green) illustrates the protection of the sarcolemma by mini-dystrophin. Monoclonal antibody immunostaining also reveals membrane leakage in injured myofibers (intense cytosolic staining, different from peripheral membrane staining of dystrophin). Inserts are high power photomicrographs of boxed areas in the corresponding panels. Arrow, immunoglobulin infiltration is prevented in transduced myofibers whereas surrounding cells were not protected. *, revertant myofibers. Scale bar, 400 μm for low power photomicrographs and 50 μm for high power inserts. (c) Quantitative evaluation of muscle pathology and muscle force in the EDL muscles described in panel b. Left panel, quantification of transduction efficiency (n = 15 for co-infection, n = 7 for acceptor only), central nucleation (n = 5 for co-infection, n = 7 for acceptor only), and lgG infiltration (n = 5 for co-infection, n = 4 for acceptor only); middle panel, specific muscle force (n = 7 pairs); right panel, resistance to eccentric contraction-induced injury (n = 7 pairs). *, the difference between co-infected muscle and single vector infected muscle was statistically significant. (d) Efficient mini-dystrophin expression in aged mdx EDL muscle moderately improved muscle function. The left EDL muscles of 1-year-old mdx mice were co-infected with 1.5 × 1010 vg particles of AV.Donor.60 and AV.Acceptor.60 (7.5 × 109 vg particles each). The right EDL muscles were mock-infected with HEPES-buffered saline. Transgene expression, muscle pathology and muscle physiology were examined 6 months later. Left panel, representative photomicrographs of the EDL muscles either co-infected with both AV.Donor.60 and AV.Acceptor.60, or mock infected with HEPES saline. Immunostaining was performed with monoclonal anti-N-terminal antibody. Scale bar, 400 μm. Right panel, resistance to eccentric contraction-induced injury (n = 3 for each group). *, the difference between co-infected muscle and saline-injected muscle was statistically significant according to paired t test. Cross, the results in BL10 muscle were significantly different from that in mdx muscle (both AAV infected and mock treated) according to one-way ANOVA and Bonferroni post hoc test. Pound, the result in BL10 was only significantly better than that of saline-injected mdx muscle according to one-way ANOVA and Bonferroni post hoc test.