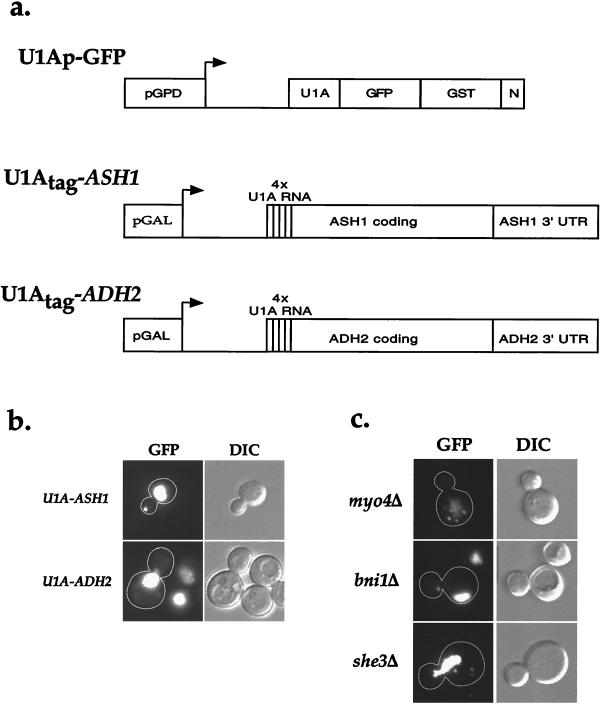
Figure 1.
Visualization of U1A-ASH1 RNA in wild-type and sheΔ cells. (a) A diagram of the constructs used to visualize and immunoprecipitate Ash1 mRNA. The RNA-binding construct (U1Ap-GFP) consisted of the amino terminus of U1A fused to GFP(S65T) for visualization, glutathione S-transferase for possible use in affinity purification, and a nuclear localization sequence (N) to lower the amount of cytoplasmic fluorescence. This construct was expressed from the constitutive glycerol phosphate dehydrogenase (GPD) promoter. To provide a binding site for U1Ap-GFP, four repeats of the U1A 3′ UTR were introduced just downstream of the start codon of either the entire coding region of ASH1 and ≈500 bp of its 3′ UTR (U1Atag-ASH1) or the entire coding region of ADH2 and ≈500 bp of its 3′ UTR (U1Atag-ADH2). Both of these constructs were expressed from the inducible galactose promoter. (b) Cells expressing U1Ap-GFP and U1Atag-ASH1 contain a single large particle at the distal tip of the bud, whereas cells expressing U1Ap-GFP and U1Atag-ADH2 display an increase in cytoplasmic fluorescence but do not contain any particles. (c) sheΔ cells contain mislocalized U1A-ASH1 RNA particles. myo4Δ and she3Δ cells contain multiple particles that are distributed throughout the mother cell, whereas bni1Δ cells contain a single particle that is localized to the neck. she2Δ and she4Δ cells displayed a similar GFP fluorescence pattern as myo4Δ and she3Δ cells (not shown).