Figure 3.
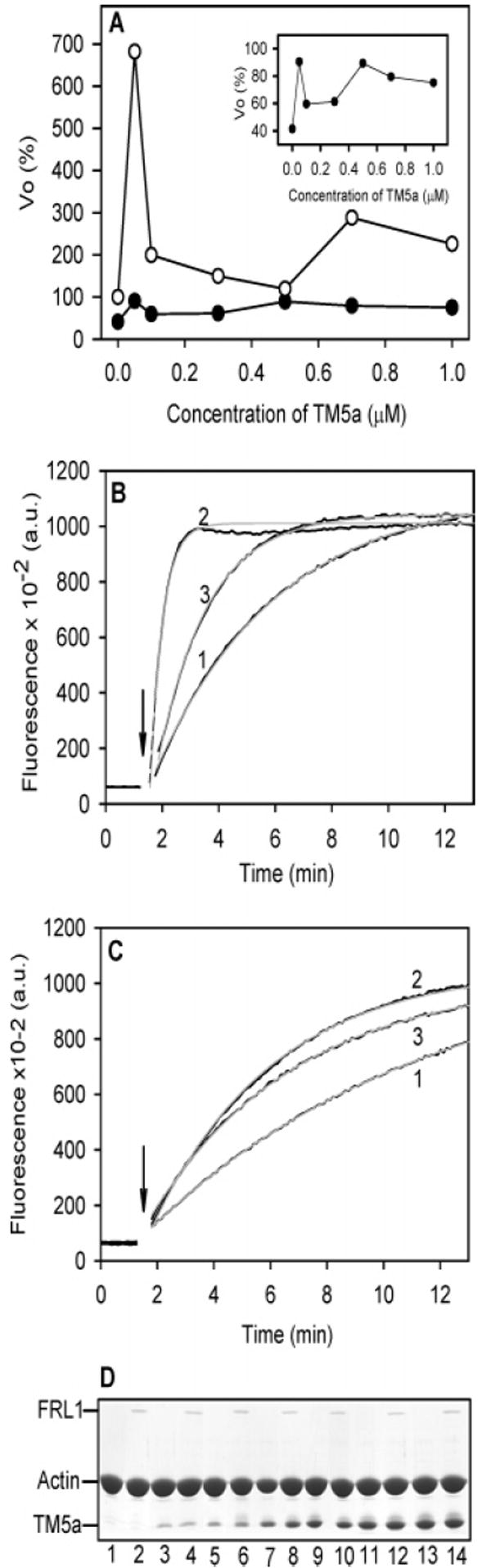
Relief of FRL1-FH1FH2 inhibition of barbed-end elongation by TM5a does not require saturation of the actin filaments with tropomyosin. The results from a representative experiment are shown; 50 nM FRL1-FH1FH2, 2 μM F-actin seeds, TM5a, varied. (A) The Vo of barbed-end elongation as a function of TM5a concentration in the absence of FRL1-FH1FH2 (○) and in the presence of 50 nM FRL1-FH1FH2 (●). The panel shows the data with FRL1-FH1FH2 on an expanded scale. (B) The time course of increase in pyrene fluorescence in the absence of FRL1-FH1FH2 in the absence of TM5a (1), a subsaturating concentration, 0.05 μM TM5a (2), and a saturating concentration, 1 μM TM5a (3). (C) The time course of increase in pyrene fluorescence in the presence of 50 nM FRL1-FH1FH2 in the absence of TM5a (1), a subsaturating concentration, 0.05 μM TM5a (2), and a saturating concentration, 1 μM TM5a (3). The gray curves are the fits to the data. (D) SDS–PAGE gels of the pellets following centrifugation at the end of the polymerization experiment. Even numbers, with FRL1-FH1FH2; odd numbers, no FRL1. TM5a concentration: 1 and 2, no TM5a; 3 and 4, 0.05 μM; 5 and 6, 0.1 μM; 7 and 8, 0.3 μM; 9 and 10, 0.5 μM; 11 and 12, 0.7 μM; 13 and 14, 1.0 μM. The ionic conditions are the same as those in Figure 1.
