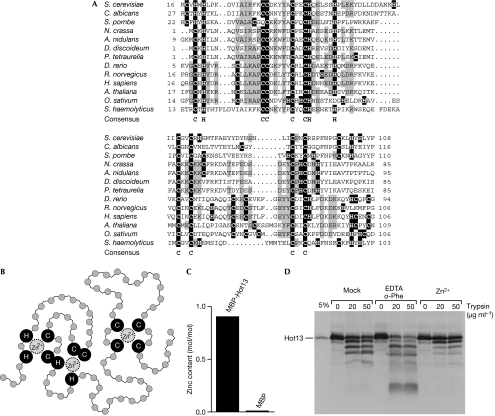
Figure 1.
Hot13 proteins form a ubiquitous protein family conserved among fungi, plants and animals. (A) Alignment of the conserved domain of Hot13 proteins. Cysteine and histidine residues are highlighted in black and conserved residues are shown in grey. The consensus line shows residues that are invariant among all Hot13 proteins. The proteins used here are: Saccharomyces cerevisiae (Hot13, AAS56591), Candida albicans (EAL02591), Schizosaccharomyces pombe (NP_596663), Neurospora crassa (EAA28094), Aspergillus nidulans (XP_001400209), Dictyostelium discoideum (EAL72440), Paramecium tetraurelia (XP_001445542), Danio rerio (AAH78283), Rattus norvegicus (AAH83739), Homo sapiens (AAH47393), Arabidopsis thaliana (BAE98773), Oryza sativum (NP_001050105) and Staphylococcus haemolyticus (YP_254069). The last protein sequence belongs to a Gram-positive bacterium and homologues of Hot13 are widely distributed in prokaryotes. (B) Model of zinc-ion coordination by conserved cysteine and histidine residues within Hot13 (pfam05495; Marchler-Bauer et al, 2007). (C) Zinc content in recombinant MBP-Hot13 and MBP as measured by inductively coupled plasma atomic emission spectroscopy. (D) Radiolabelled Hot13 was synthesized in reticulocyte lysate and pretreated in the absence or presence of 5 mM EDTA, 1 mM o-phenanthroline or 10 μM zinc acetate. Trypsin was added at the indicated concentrations for 30 min on ice. Hot13 was visualized by autoradiography. MBP, maltose-binding protein.