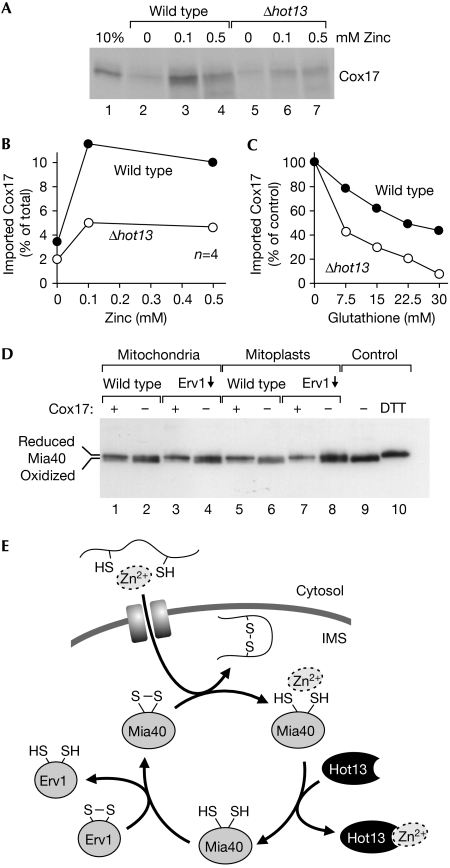
Figure 5.
The import of purified Cox17 shifts Mia40 into its reduced conformation. (A) Recombinant Cox17 was expressed in the presence of 35S-sulphate and purified. Cox17 (12.5 pmol) was incubated with isolated wild-type or Δhot13 mitochondria in the presence of 14 mM glutathione and the indicated zinc concentrations at 25°C. After 10 min, the mitochondria were treated with proteinase K to remove non-imported protein, re-isolated, washed and subjected to SDS–PAGE. For a control, 10% of the Cox17 used per import reaction was loaded in lane 1. (B) The experiment shown in (A) was repeated four times. The radioactive signals were quantified and expressed in relation to the total Cox17 protein added per reaction. The mean values are shown. (C) Recombinant Cox17 was imported into mitochondria in the presence of 0.5 mM zinc and increasing concentrations of glutathione. After autoradiography, the imported protein was quantified and expressed in comparison with the amount of protein imported in the absence of glutathione. (D) Recombinant Cox17 was expressed in the presence of 200 μM zinc acetate and purified. The protein was incubated with mitochondria or mitoplasts from wild-type or Erv1-depleted yeast strains for 10 min. For a control, the samples were incubated with elution buffer. Samples were analysed as in Fig 2B. (E) Model for the influence of metal ions on protein import into the IMS. Imported proteins can be associated with zinc ions. These are passed through Mia40 to Hot13 and further to an unknown acceptor. Hot13-mediated demetalation of Mia40 presumably improves the reoxidation of the import receptor by Erv1. IMS, intermembrane space; SDS–PAGE, SDS–polyacrylamide gel electrophoresis.