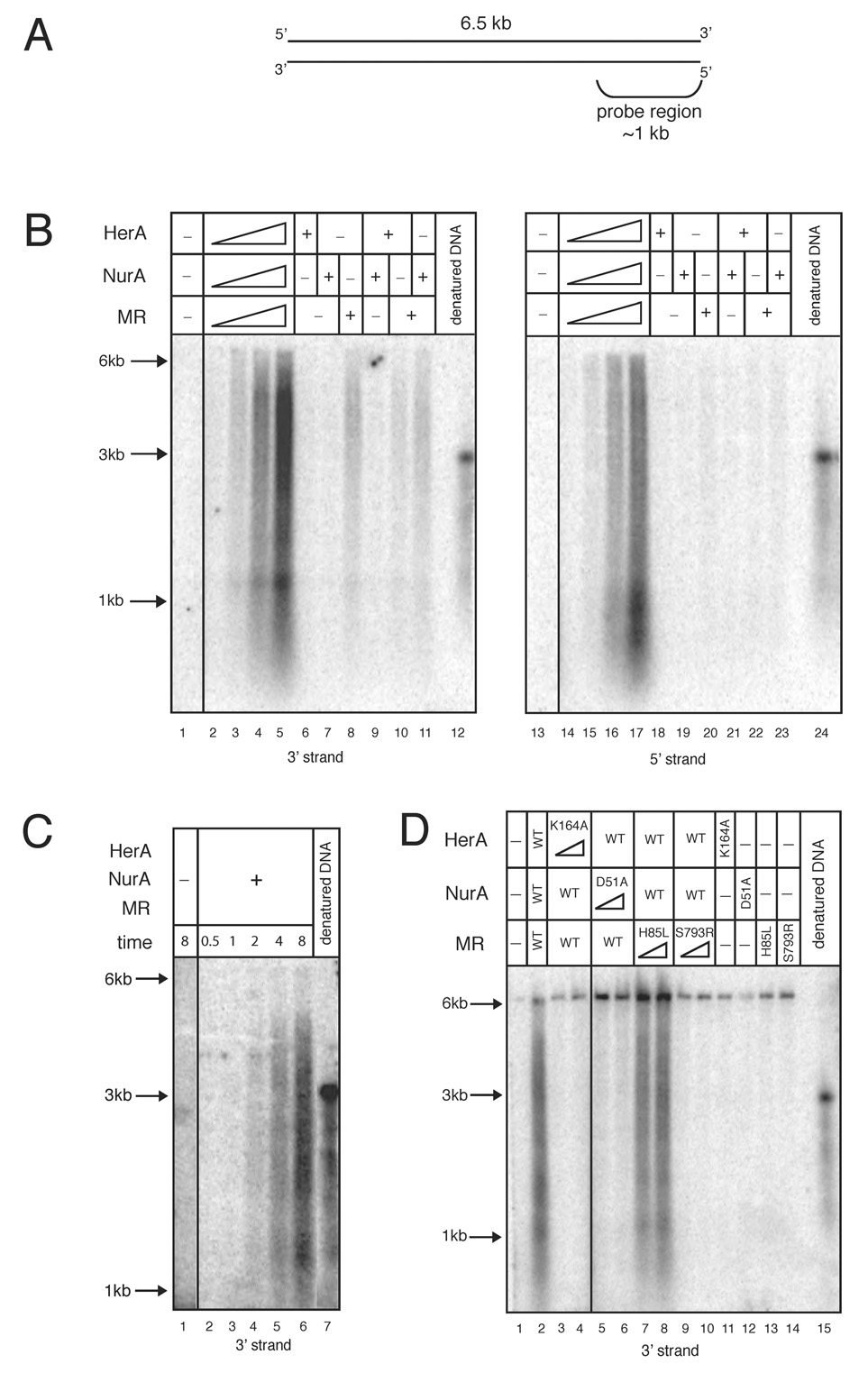
Figure 2. Mre11, Rad50, HerA, and NurA function cooperatively to resect the 5’ strand of a DSB.
(A) Schematic of the linearized plasmid substrate used in nuclease reactions analyzed by Southern hybridization. The DNA length and location of the probe region are indicated.
(B) Nuclease assays were performed with a 6.5 kb linearized plasmid DNA substrate and analyzed by non-denaturing Southern hybridization with strand-specific RNA probes. Reactions contained 0.3, 1.4, 2.7, and 5.4 nM wild-type HerA, 1.9, 9.5, 19.2, and 38.4 nM wild-type NurA, and 0.3, 1.7, 3.3, and 6.6 nM wild-type MR in 5 mM MgCl2, 1 mM ATP, and 100 mM NaCl. “+” indicates 5.4 nM HerA, 38.4 nM NurA, and 6.6 nM MR wild-type proteins. Reactions were incubated at 65°C for 15 min and analyzed in native agarose gels. The denatured DNA blot control consists of NaOH-denatured substrate DNA, which runs at a position equivalent to ~3 kb non-denatured dsDNA. DNA molecular weight ladder positions of 6, 3, and 1 kb are indicated. The probe used in the left panel recognizes the 3’ strand on one end of the DNA, while the probe used in the right panel recognizes the 5’ strand.
(C) Time course reaction as in (B) with 5.4 nM HerA, 38.4 nM NurA, and 6.6 nM MR wild-type proteins. Reactions were stopped at 0.5, 1, 2, 4, or 8 mins and analyzed with the 3’ strand-specific probe.
(D) Nuclease assays as in (B) but with 5.4 or 10.8 nM HerA K164A, 38.4 or 76.8 nM NurA D51A, and 6.6 or 13.2 nM M(H85L)R and MR(S793R) as indicated. “WT” indicates 5.4 nM HerA, 38.4 nM NurA, and 6.6 nM MR wild-type proteins. Reactions were analyzed with the 3’ strand-specific probe.