Abstract
Protein E, the lysis protein of bacteriophage φX174, is a specific inhibitor of MraY, the phospho-MurNAc-pentapeptide translocase that catalyzes the synthesis of lipid I in the conserved pathway for peptidoglycan biosynthesis. The original evidence for this inhibition was the isolation of two spontaneous E-resistance mraY mutants. Here we report further genetic studies aimed at dissecting the interaction between E and MraY, using a genetic strategy that is facile, rapid, and does not depend on the availability of purified E, purified MraY, or its substrates. This system relies on the ability of mraY or its enzymatically inactive D267N allele to protect cells from lysis after induction of a chimeric λ∷E prophage. Using this approach, the MraY protein from Bacillus subtilis, which shares 43% sequence identity with the Escherichia coli enzyme, was found to interact weakly, if at all, with E. A potential E binding site defined by transmembrane domains 5 and 9 has been identified by isolating more mraY mutants resistant to E inhibition. Genetic analysis indicates that these E-resistant alleles fall into three classes on the basis of the affinity of the encoded proteins for MraY.
IN infections of double-strand DNA phages, host lysis is a strictly regulated, precisely timed, multigenic event, involving up to five proteins, including a holin to permeabilize the cytoplasmic membrane and an endolysin to degrade the cell wall(Young et al. 2006). In contrast, host lysis by the much simpler single-strand RNA (ssRNA) and DNA (ssDNA) phages is accomplished by expressing a single gene with no known relationship to any of the lysis genes encoded by more complex phage (Henrich et al. 1982; Young and Young 1982; Coleman et al. 1983; Karnik and Billeter 1983; Winter and Gold 1983; Bernhardt et al. 2002b). There are three unrelated lysis genes encoded by these small phage: E, in the prototype microvirus (ssDNA) φX174; A2, in the prototype allolevirus (ssRNA) Qβ; and L, in the prototype levivirus (ssRNA) MS2. Although the mechanism of lysis mediated by L remains obscure, it has been established that both E and A2 operate by inhibiting cytoplasmic steps in cell wall synthesis (Bernhardt et al. 2000, 2001a,b).
E has had a prominent role in the history of molecular biology. It was the first gene shown to be completely embedded within another gene in a different reading frame (Sanger et al. 1977) (Figure 1) and was the first gene to be subjected to site-directed mutagenesis (Hutchison et al. 1978). E encodes a 91-amino-acid protein that is encoded by >90% of φX174 mRNAs (Hayashi et al. 1976) and is localized to the cytoplasmic membrane, presumably by virtue of its putative N-terminal transmembrane domain (TMD) (Altman et al. 1983; Bläsi et al. 1983). Gene fusion experiments have shown that only the N-terminal 35 amino acids of E, including its putative TMD, are required for its lytic activity (Maratea et al. 1985; Buckley and Hayashi 1986). Moreover, EΦβ–galactosidase fusions are lytically active and exhibit β-galactosidase activity, indicating that E has an N-out, C-in topology. We have shown that E causes lysis in growing cells by blocking cell wall synthesis and that this blockage is effected by specific inhibition of MraY, a conserved enzyme in the pathway for murein biosynthesis (Bernhardt et al. 2000, 2001a). MraY, also known as translocase I, catalyzes the formation of the precursor lipid I by transferring phospho-MurNAc-pentapeptide from UDP-MurNAc-pentapeptide to undecaprenol-P. MraY has been proposed to have 10 TMDs and to adopt an N-out, C-out topology (Bouhss et al. 1999) (Figure 1). Lloyd et al. (2004) have shown that aspartate residues at positions 115, 116, and 267 are essential for MraY activity in vitro. All three of these residues would reside in cytoplasmic loops of MraY given its predicted topology. D115 and D116 are thought to coordinate the Mg2+ ion involved in binding the pyrophosphate moiety of the UDP-MurNAc-pentapeptide substrate, while D267 is predicted to be an active-site nucleophile that attacks its β-phosphate.
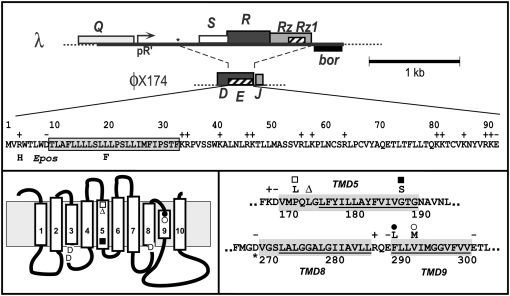
Figure 1.—
Features of E and MraY. Top: Structure of the lysis gene regions of the phages λ and φX174, showing the replacement of SRRzRz1 with E in the chimera λ*E used in this study. The position of a mutation that increases expression of E in this chimera is indicated by an asterisk (Zheng et al. 2008) and the two missense changes in Epos are shown at the bottom of the primary structure of E (Bernhardt et al. 2002a). Bottom left: Proposed topology of MraY, based on primary structure analysis using the MemBrain algorithm (Shen and Chou 2008), is slightly modified from the topology of Bouhss et al. (1999), mainly in the the placement of TMDs 1, 2, and 5. This topology is still consistent with the results of the β-lactamase fusion study upon which the original topology was based. The beginning and ending residues for each TMD in this model, compared, where different, with those of the previous model, in parentheses, are as follows: TMD1, 25–42 (19–45); TMD2, 70–92 (77–90); TMD3, 97–113; TMD4, 134–153 (134–156); TMD5, 168–188 (174–188); TMD6, 200–220; TMD7, 234–257 (239–251); TMD8, 268–284 (271–284); TMD9, 288–299; and TMD10, 342–358 (343–358). The positions of three conserved Asp residues important for enzyme activity are indicated on cytoplasmic loops at the bottom of TMD3 and TMD8 (Lloyd et al. 2004). The positions of mraY mutations conferring resistance to E are indicated by: Δ, ΔL172, and •, F288L, reported previously (Bernhardt et al. 2000); and □, P170L; ▪, G186S; and ○, V291M. Bottom right: The sequences of two regions of MraY in which E-resistance mutations have been isolated are shown, with the proposed catalytic Asp267 residue indicated by an asterisk. The extent of the TMDs proposed in this study and in the previous work is indicated by shaded rectangles and underlining, respectively.
In our original study, MraY was identified as the target of E by the isolation of two dominant mraY mutations conferring resistance to this lysis protein. One of these was a single-codon deletion, ΔL172, in putative TMD5 and the other a missense change, F288L, in putative TMD9 (Figure 1). Here we report studies extending the mutational analysis of the E–MraY interaction and discuss the results in terms of a model for the E-mediated inhibition of MraY.
MATERIALS AND METHODS
Media, chemicals, and general methods:
Cultures were grown in standard LB media supplemented with appropriate antibiotics, as described (Tran et al. 2005). Inductions were performed by addition of arabinose to a final concentration of 0.2% and, for lysogenic cultures, beginning 2 min after arabinose induction, by aerating at 42° for 15 min and at 37° thereafter. Lysis profiles were obtained by monitoring A550 after induction, as described previously (Ramanculov and Young 2001). β-galactosidase activity was assayed according to Miller (1972b), except that the cells are pelleted and resuspended in assay buffer, as described by M. Price-Carter (unpublished data) (http://rothlab.ucdavis.edu/protocols/beta-galactosidase-3.html). Plasmid DNA isolation, DNA amplification by PCR, DNA transformation, DNA sequencing, and Quikchange (Stratagene) site-directed mutagenesis were performed as previously described (Tran et al. 2005).
Bacterial strains, bacteriophages, and plasmids:
The prototroph MDS12 tonA∷Tn10 (Kolisnychenko et al. 2002; Tran et al. 2005), carrying deletions of all the cryptic prophage sequences of E. coli, was used as the host for all lysogenic inductions. The phages λ*E and λEpos (Figure 1) and the construction of single-copy lysogens have been described (Zheng et al. 2008). The medium-copy plasmid pMY30 has the E. coli mraY gene (EcmraY) inserted between the SmaI and HindIII sites of pBAD30 (Guzman et al. 1995) placing it under the control of the paraBAD promoter (Bernhardt et al. 2000). The plasmid pBAD30-BsMraY was constructed similarly except the mraY gene was from B. subtilis W23 (nt 1587210–1588202 of the B. subtilis genome). A strain with a chromosomal ΔmraY was constructed using the protocol of Link et al. (1997). Briefly, the plasmid pKOMY3 was constructed by inserting a DNA fragment spanning nt 95015–98343 of the E. coli K-12 genome into the unique BamHI site of pKO3, a vector with a ts-replicon. This construct carries mraY as well as portions of the upstream and downstream genes murF and murD. Next the plasmid pKOMY3ΔmraY was constructed, in which the entire mraY sequence was deleted (nt 96025–97051 of the E. coli genome), leaving 1 and 1.3 kb of homology upstream and downstream of ΔmraY, respectively. The strain RY3316 was constructed from MG1655 (F− ilvG rfb50 rph1; obtained from the E. coli Genetic Stock Center http://cgsc.biology.yale.edu/) by exchanging the deletion from pKOMY3ΔmraY into the chromosome, as described (Link et al. 1997). The strain RY3321, which is RY3316 recA srl∷Tn10 pKOMY3, was constructed by P1 transduction and used as the host strain for all complementation experiments. To test the ability of each allele of mraY to functionally replace the wild-type (wt) gene in E. coli, we first placed it under the control of the ara promoter in the vector pBAD30 and transformed the resulting plasmid into RY3321. The ability of the transformants to grow at 42° in the presence, but not the absence, of arabinose was taken as proof that the mraY gene on the pBAD30 plasmid was able to complement a chromosomal mraY deletion.
Selection of mraY mutants resistant to E:
Mutants resistant to E-mediated lysis were isolated as previously described (Bernhardt et al. 2000), except that the cells were mutagenized with ethylmethanesulfonate (EMS), essentially as described by Miller (1972a), prior to the selection. The only differences in the protocol used here were that the exposure to EMS was limited to 15–30 min, instead of 2 hr, and that, after the EMS treatment, the cells were washed twice in minimal salts, grown in LB overnight, and stored at −80° after addition of dimethylsulfoxide (85 μl/ml of culture). Individually treated cultures were tested for the frequency of rifampicin resistance as a measure of mutagenesis. Cultures treated for 15 and 30 min exhibited ∼60-fold and 100-fold increases in rifampicin resistance, respectively, and were subcultured and used for selection.
Detection of MraY:
Bethyl Laboratories (Montgomery, TX) prepared the antibody used for detecting MraY by affinity purification of antisera raised against the peptide RGQRIFRMAPIHHHYEL (residues 314–330 of MraY). For the detection of MraY, logarithmic cultures of MDS12 tonA∷Tn10 were induced at A550 = ∼0.6. After 1 hr, cells were harvested by centrifugation and 1 A550 unit was analyzed by SDS–PAGE on a 12% separating gel and immunoblotting, as described (Zheng et al. 2008).
RESULTS
Overexpression of active and inactive alleles of E. coli mraY protects against E-mediated lysis:
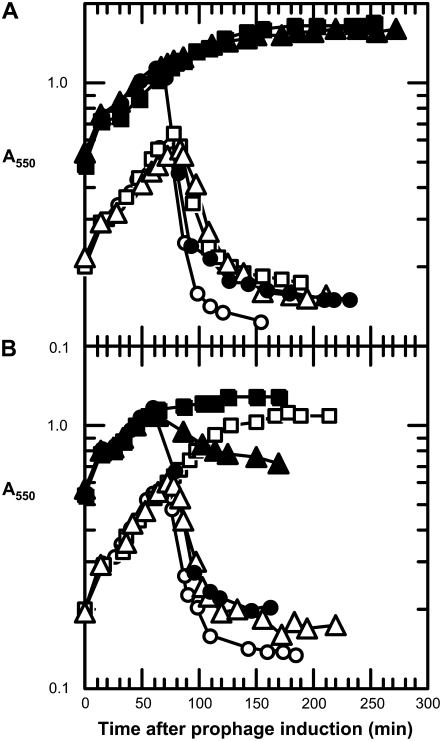
Since E is an inhibitor of MraY, it seemed likely that the overexpression of mraY would prevent E-mediated lysis. This hypothesis was tested in a “protection assay” using a host with a copy of the mraY gene on the chromosome carrying the E. coli mraY gene (EcmraY) on an arabinose-inducible plasmid, pBAD30 in trans to a thermally inducible λ prophage, λ*E, in which the lysis gene cassette is replaced by the E gene (Zheng et al. 2008). When a culture of this strain was sequentially induced with arabinose and a thermal shift in early logarithmic phase (A550 = 0.2), the EcmraY plasmid had no effect on lysis (Figure 2). However, when cultures were induced at a higher culture density, lysis was prevented. Presumably, this reflects increased EcmraY expression from the plasmid at higher culture densities because of higher cAMP levels and the catabolite-sensitive character of the arabinose promoter of pBAD30 (Guzman et al. 1995). This interpretation is supported by the finding that the lacZ expression is 2.5-fold higher at A550 = 0.5 than at A550 = 0.2 (not shown).
Figure 2.—
Induction of plasmid-borned mraY alleles can protect against E-mediated lysis. Cultures of MDS12 tonA∷Tn10 (λ*E), bearing derivatives of the plasmid pBAD30 carrying the indicated alleles of mraY of E. coli (A) or B. subtilis (B) were induced at either A550 = 0.2 (open symbols) or A550 = 0.5 (solid symbols) and monitored for culture turbidity, as described in materials and methods. ○ and •, pBAD30 vector; □ and ▪, pBAD30 carrying the wt mraY gene; and ▵ and ▴, pBAD30 carrying the inactivated gene (EcmraYD267N or BsmraYD231N).
Next, we tested a catalytically inactive allele of EcmraY for its ability to similarly prevent lysis of the induced λ*E lysogen. For this experiment, we used the EcmraYD267N allele, which encodes an inactive protein thought to be defective because the D267N missense change eliminates a putative active-site nucleophile (Lloyd et al. 2004). Once again, arabinose induction at low cell densities had no effect on lysis, but at higher cell densities, lysis was completely blocked (Figure 2). This suggests that, if produced in sufficient quantity, an inactive MraY protein can bind enough of the E protein produced by the induced λ*E lysogen to spare functional MraY, produced from the chromosomal mraY gene, from E-mediated inhibition.
A heterologous MraY protein does not interact with E:
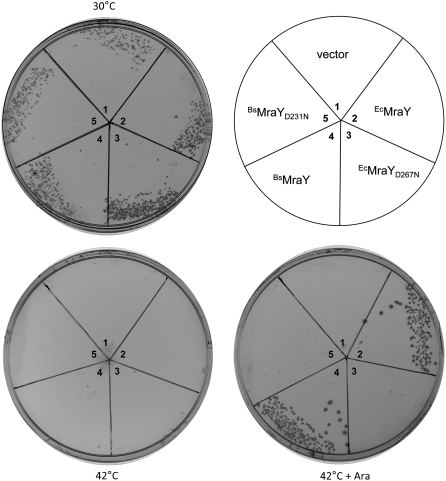
Since the MraY proteins from gram-positive bacteria diverge significantly when compared to EcMraY, the specific protein–protein contacts necessary for E-sensitivity might not occur with enzymes from the former. In fact, it has been reported that the cloned E gene is not lytic when expressed in Staphylococcus carnosus (Halfmann et al. 1993). To assess the ability of the plasmid-based system to discriminate between MraY proteins on the basis of their interaction with E, we decided to repeat the experiments described above using plasmids carrying active and inactive alleles of the mraY gene from B. subtilis (BsmraY). As a first step, we tested the ability of the BsmraY gene to complement a chromosomal deletion of mraY. In this experiment, the only source of MraY protein is from the transformed plasmid, unlike the protection assay where the chromosomal mraY is kept intact for testing the ability to protect by the enzymatically inactive allele. As can be seen in Figure 3, the essential function(s) of MraY in E. coli can be fulfilled by the B. subtilis enzyme. Since sequence alignment indicates that Asp231 of the BsMraY is equivalent to the proposed catalytic Asp267 of the EcMraY (Lehrman 1994; Al-Dabbagh et al. 2008), we tested both EcmraYD267N and BsmraYD231N genes for their ability to complement the mraY deletion. As can be seen in Figure 3, neither allele allowed cell growth at the restrictive temperature, consistent with a catalytic role for the altered aspartate residues protein.
Figure 3.—
BsmraY complements ΔEcmraY. RY3321, which has a chromosomal deletion of mraY and carries the wt EcmraY gene on a low-copy, ts-replicon, was transformed with the indicated plasmids and tested for growth under the indicated conditions, as described in materials and methods.
We next examined the ability of BsmraY and BsmraYD231N to prevent lysis of an induced λ*E lysogen. In contrast to what was observed with EcmraY, the ability of the active allele, BsmraY, to block E-mediated lysis was independent of culture density (Figure 2) suggesting that fewer molecules of the Bacillus enzyme are required to protect cells from lysis after induction of λ*E. However, overexpression of the catalytically inactive allele, BsmraYD231N, afforded little or no protection against E-mediated lysis in this system. Taken together, these results suggest that the active B. subtilis enzyme, even at the lower level of production, can provide sufficient lipid I, despite inhibition of the host MraY by E, but the inactive BsMraY, even at the higher level of expression, cannot titrate out E and thus allow lipid I production by the host MraY. Thus, in our protection assay, the EcmraY and BsmraY genes are useful as controls encoding proteins that, respectively, do and do not interact strongly with E. For the experiments described below, the ability of the inactivated version of any mraY allele to protect against lysis is taken as evidence for the ability of its product to bind E and thus spare the host MraY activity.
The E-resistant alleles of EcmraY encode proteins with different apparent affinities for E:
The original E-resistant mutants were obtained by inducing a plasmid-borne allele of E and then screening the spontaneous survivors for resistance to φX174 (Bernhardt et al. 2000). More than 99% of the survivors harbored alterations in the E-plasmid, and only two φX174R alleles of mraY were found, ΔL172 and F288L. To increase the pool of φX174R alleles, we used EMS mutagenesis to increase the total frequency of survivors by ∼20-fold. Every phage-resistant isolate was found to have a missense change in the mraY gene. However, from three independent mutagenesis pools in which a total of 13 mutants were sequenced, only three more alleles were obtained, all multiple times (not shown). Together with the original mutants used to identify MraY as the target of E, the five E-resistance mutations mapped to only 2 of the 10 TMDs of MraY (Figure 1). The clustering of these mutations in the 2 TMDs and their repeated isolation suggested that this mutant selection was at or near saturation.
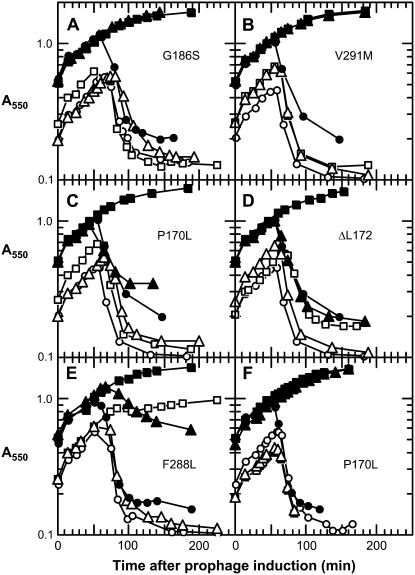
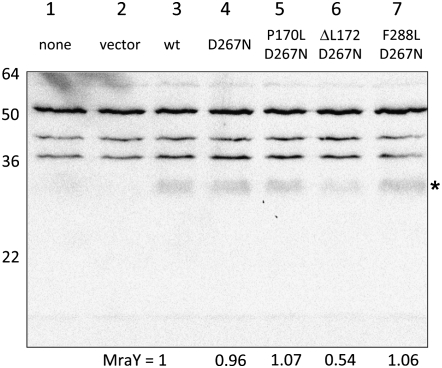
We examined the behavior of the five E-resistant alleles of EcmraY in our protection assay. As can be seen in Figure 4, these alleles fall into three classes. Two of these mraY mutants, G186S and V291M, are indistinguishable from the wild type. In their active or inactive (D267N) forms, neither protects at low culture density but both do at high culture density. Alleles encoding the P170L and ΔL172 variants protect only in their active forms and only at high culture density. Finally, the EcMraYF288L protein appears to be similar to BsMraY in that protection is observed only with the active enzyme but occurs at both low and high culture densities. Although the low levels of MraY even from the plasmid-borne alleles preclude accurate quantitation by immunoblot, nevertheless it is clear that at least for the mraYP170L,D267N and mraYF288L,D267N alleles, the amount of MraY protein in these protection experiments is as high as or higher than the parental mraYD267N (Figure 5).
Figure 4.—
E-resistant alleles of mraY show different protection against E-mediated lysis. Inductions and symbols are the same as in Figure 2, except that the indicated EcmraY allele is used; in F, the prophage is λEpos. ○ and •, pBAD30 vector; □ and ▪, pBAD30 carrying mraY; and ▵ and ▴, pBAD30 carrying the inactivated gene (mraYD267N).
Figure 5.—
Accumulation of MraY proteins. Membranes from induced cultures bearing no plasmid (lane 1), pBAD30 (lane 2), or pBAD30 carrying the indicated allele of EcmraY (lanes 3–7) were analyzed by immunoblot with antibodies raised against a peptide of MraY. The position of molecular mass standards are indicated to the left and the relative amount of MraY, by integration of the band indicated by an asterisk, is given at the bottom of lanes 4–7, relative to the amount in lane 3.
MraYP170L interacts with Epos more strongly than E:
The alleles of EcmraY that provide resistance to E were originally selected using a plasmid encoding the Epos gene (Bernhardt et al. 2000). This was necessary because otherwise frequent knockout mutations in slyD, which encodes an abundant cytoplasmic peptidyl-prolyl isomerase required for the stability of the E protein, would overwhelm the selection for E insensitivity (Maratea et al. 1985; Roof et al. 1994; Bernhardt et al. 2002a). The Epos allele does not require slyD for function. The protein encoded by the Epos gene has two missense changes, R3H and L19F (Figure 1). For this reason, we repeated the protection assays using a λEpos lysogen. The only significant difference from the experiments using the λ*E lysogen was found with the EcmraYP170L plasmid. While the inactive form (D267N) of EcMraYP170L did not protect against lysis by E under any condition tested, it did provide complete protection against Epos at high culture densities (Figure 4). The simplest interpretation of this result is that EcMraYP170L binds Epos more tightly than E.
DISCUSSION
Genetic systems for assessing mraY function and interaction with E:
Here, we present two genetic systems for the further analysis of MraY and its interaction with E, the lysis protein of φX174. First, using a host with a deletion of mraY on the chromosome and a functional copy of mraY on a ts-replicon, we were able to test the ability of any given mraY allele to substitute for the EcmraY gene. Somewhat unexpectedly, we found that the highly divergent BsmraY gene was able to complement a chromosomal EcmraY deletion. Given the high degree of divergence in the primary structures of EcMraY and BsMraY, this argues that the single, essential function of MraY is to convert UDP-MurNAc-pentapeptide into lipid I. It has been suggested that MraY might participate in the formation of a multienzyme complex or “machine” that is essential for the biosynthesis of peptidoglycan (Bugg et al. 2006; Mendel et al. 2006; Bouhss et al. 2008). While our results do not rule out this possibility, they do suggest that MraY is, at best, a peripheral and not essential for the assembly of such a machine. Finally, we found that the EcmraYD267N allele was unable to complement the chromosomal mraY deletions, providing additional support for the proposal that Asp267 is an essential residue for EcMraY, as reported by Lloyd et al. (2004). That this system provides a robust, low-background readout on solid medium for functional determination makes it ideally suited for high-throughput analysis of randomly mutated mraY, which, to date, has been subjected only to limited site-directed mutagenesis of conserved residues (Lloyd et al. 2004). The adjustability of the paraBAD vector, using alleles of pcnB to alter copy number (Lopilato et al. 1986) and numerous agents that exert different levels of catabolite repression also may allow this system to be used for a chemical biology approach in screening for small molecule inhibitors of MraY.
In a second type of assay, the ability of a plasmid-borne allele of mraY under paraBAD control is tested for its ability to prevent the lysis by an induced λ*E lysogen. The chromosomal mraY gene of the host is left intact, so that even enzymatically inactive mraY alleles could be examined for their ability to protect against E-mediated lysis. When EcmraY was present on the plasmid, we found that inductions of a culture in early logarithmic phase had no effect on lysis, but when cultures were allowed to grow to a higher culture density before induction, lysis was prevented. Presumably, this dependence on culture density reflects the 2.5-fold higher level of expression at high culture density from the catabolite-sensitive paraBAD promoter in the plasmid vector. Essentially identical results were obtained when the EcmraYD267N allele was present on the plasmid. We interpret this as indicating that the inactive MraYD267N protein bound E and, thus, spared a smaller pool of active MraY produced from the chromosomal mraY gene from E-mediated inhibition. Strikingly different results were obtained using plasmids carrying BsmraY or a variant encoding an inactive protein, BsmraYD231N. First, the protection seen with BsmraY was independent of culture density, suggesting that the BsMraY protein protected against E-mediated lysis, even when present at low levels, by catalyzing the formation of lipid I. Furthermore, in contrast to what was seen with EcmraYD267N, induction of BsmraYD231N did not block lysis at either high or low cell densities. Together, these results indicate that the BsMraY protein has very low affinity, if any, for E. Thus, the protection assay we have developed is a genetic tool that can be used to distinguish between genes encoding MraY proteins with differing apparent affinities for E.
Next, we assessed the behavior of five EcmraY alleles that provide resistance to φX174 in our protection assay (Figure 4; Table 1). Two of these alleles were previously identified (Bernhardt et al. 2000), while the isolation of the other three is described in this study. The protection assay allowed these five alleles to be divided into three classes, on the basis of their apparent affinity for E. The G186S and V291M variants behaved identically to wild-type EcmraY, in that their catalytically active and inactive forms protect at the higher expression level, but not at the lower expression level (Figure 4, A and B). In contrast, the F288L mutant was indistinguishable from BsmraY, protecting at low expression when catalytically active but unable to protect in the catalytically inactive form even when produced at the higher level (Figure 4E, open squares). We interpret this as indicating that F228L abrogates or severely reduces E binding, because the active form can provide lipid I even in the presence of excess E and an excess of its inactive form does not titrate E. The other two alleles, P170L and ΔL172, exhibited an intermediate behavior, since their active forms resemble wt EcmraY in requiring the higher expression level to prevent lysis, but their inactive forms, like BsMraYD231N, cannot titrate E when overexpressed (Figure 4, C and D, solid triangles). Thus, in this interpretation, P170L and ΔL172 would have an intermediate affinity for E. All five of the φX174R alleles of mraY were initially isolated by selecting for cells that survived the induction of the cloned Epos gene, which has two changes, R3H and L19F, relative to wild-type E. Thus, it was satisfying to find that the protection assay indicated that one of the φX174R variants, EcMraYP170L, interacts more strongly with Epos than it does with E. Although preliminary in nature, this observation might indicate that one or both of the residues altered in Epos interacts with Leu170 of EcMraYP170L. Moreover, since all five E-resistance mutations map to predicted TMDs 5 and 9 of MraY, it is tempting to speculate that they interact directly with the single TMD of E. In the continued absence of any structural information about MraY, using a genetically malleable probe like E may be an effective way to make progress toward mechanistic understanding. From this perspective, we note that the detailed topology that we have presented for MraY (Figure 1) differs from that proposed by Bouhss et al. (1999) in the positions of the TMDs, most specifically to allow the sites that give rise to E resistance to be contained within domains predicted to span the bilayer.
TABLE 1.
The ability of plasmid-borne mraY alleles to protect against E-mediated lysis defines different levels of E-binding
| MraY protein | Position of E-resistance mutation | Protection at A550 = 0.2 | Protection at A550 = 0.5 | Apparent affinity for E |
|---|---|---|---|---|
| MraY | No | Yes | +++ | |
| MraYD267N | No | Yes | ||
| BsMraY | Yes | Yes | −/+ | |
| BsMraYD231N | No | No | ||
| MraYG186S | TMD5 | No | Yes | +++ |
| MraYG186S, D267N | No | Yes | ||
| MraYV291M | TMD9 | No | Yes | +++ |
| MraYD267N, V291M | No | Yes | ||
| MraYΔL172 | TMD5 | No | Yes | + |
| MraYΔL172, D267N | No | No | ||
| MraYP170L | TMD5 | No | Yes | + |
| MraYP170L, D267N | No | No | ||
| MraYF288L | TMD9 | Yes | Yes | −/+ |
| MraYD267N, F288L | No | No |
Implications for the mechanism of E inhibition:
The ability of BsmraY to complement chromosomal ΔmraY casts doubt on the notion that MraY plays an integral role in the formation of a multiprotein machine required for murein synthesis and suggests, instead, that its sole essential role is to catalyze the formation of lipid I. This perspective is also inconsistent with the model proposed by Mendel et al. (2006) where E acts by binding MraY and preventing its incorporation into such a complex. Our results do put constraints on models for the E-mediated inhibition of MraY. First, the ability of modest increases in expression of mraY to block E-mediated lysis indicates that E does not function catalytically, like some bacteriocins of approximately the same size. Host lysis by λ*E occurs in approximately the same time scale after infection as occurs with φX174, so the level of E produced is likely to be comparable in the two cases. This suggests that φX174 does not produce E in large excess over its target, MraY, presumably because the phage never encounters situations where the level of MraY is dramatically different. Together, the protection conferred by the catalytically inactive protein in sparing the chromosomal MraY and the existence of three classes of E-resistant MraY mutants may indicate that the φX174R alleles of mraY encode proteins with different affinities for E. In this view, the F288L mutant is resistant because it binds E poorly, whereas the G186S and V291M mutants bind E with an affinity that is not distinguishable, at least in our assay, from that of the wt protein. These classes resemble the different classes of inducer insensitivity that have been observed in the Lac repressor, in which some mutations block inducer binding but others interfere with the inducer-mediated conformational change (Pace et al. 1997). It will be interesting to exploit this system to select E mutants that overcome the mraY mutations, with the aim of using allele-specific suppression to map out point-to-point interactions between E and MraY.
Acknowledgments
We thank the members of the Young laboratory, past and present, for their helpful criticisms and suggestions. This work was supported by Public Health Service grant GM27099 to R.Y., the Robert A. Welch Foundation, and the Program for Membrane Structure and Function, a Program of Excellence grant from the Office of the Vice President for Research at Texas A&M University.
References
- Al-Dabbagh, B., X. Henry, M. El Ghachi, G. Auger, D. Blanot et al., 2008. Active site mapping of MraY, a member of the polyprenyl-phosphate N-acetylhexosamine 1-phosphate transferase superfamily, catalyzing the first membrane step of peptidoglycan biosynthesis. Biochemistry 47 8919–8928. [DOI] [PubMed] [Google Scholar]
- Altman, E., R. K. Altman, J. M. Garrett, R. J. Grimaila and R. Young, 1983. S gene product: identification and membrane localization of a lysis control protein. J. Bacteriol. 155 1130–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt, T. G., W. D. Roof and R. Young, 2000. Genetic evidence that the bacteriophage φX174 lysis protein inhibits cell wall synthesis. Proc. Natl. Acad. Sci. USA 97 4297–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt, T. G., D. K. Struck and R. Young, 2001. a The lysis protein E of φX174 is a specific inhibitor of the MraY-catalyzed step in peptidoglycan synthesis. J. Biol. Chem. 276 6093–6097. [DOI] [PubMed] [Google Scholar]
- Bernhardt, T. G., I. N. Wang, D. K. Struck and R. Young, 2001. b A protein antibiotic in the phage Qβ virion: diversity in lysis targets. Science 292 2326–2329. [DOI] [PubMed] [Google Scholar]
- Bernhardt, T. G., W. D. Roof and R. Young, 2002. a The Escherichia coli FKBP-type PPIase SlyD is required for the stabilization of the E lysis protein of bacteriophage φX174. Mol. Microbiol. 45 99–108. [DOI] [PubMed] [Google Scholar]
- Bernhardt, T. G., I. N. Wang, D. K. Struck and R. Young, 2002. b Breaking free: “protein antibiotics” and phage lysis. Res. Microbiol. 153 493–501. [DOI] [PubMed] [Google Scholar]
- Bläsi, U., R. Geisen, W. Lubitz, B. Henrich, R. Plapp et al., 1983. Localisation of the bacteriophage φX174 lysis gene product in the cell envelope of Escherichia coli, pp. 205–210 in The Target of Penicillin. Walter de Gruyter, Berlin.
- Bouhss, A., D. Mengin-Lecreulx, D. Le Beller and J. van Heijenoort, 1999. Topological analysis of the MraY protein catalysing the first membrane step of peptidoglycan synthesis. Mol. Microbiol. 34 576–585. [DOI] [PubMed] [Google Scholar]
- Bouhss, A., A. E. Trunkfield, T. D. Bugg and D. Mengin-Lecreulx, 2008. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol. Rev. 32 208–233. [DOI] [PubMed] [Google Scholar]
- Buckley, K. J., and M. Hayashi, 1986. Lytic activity localized to membrane-spanning region of φX174 E protein. Mol. Gen. Genet. 204 120–125. [DOI] [PubMed] [Google Scholar]
- Bugg, T. D., A. J. Lloyd and D. I. Roper, 2006. Phospho-MurNAc-pentapeptide translocase (MraY) as a target for antibacterial agents and antibacterial proteins. Infect. Disord. Drug Targets 6 85–106. [DOI] [PubMed] [Google Scholar]
- Coleman, J., M. Inouye and J. Atkins, 1983. Bacteriophage MS2 lysis protein does not require coat protein to mediate cell lysis. J. Bacteriol. 153 1098–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman, L. M., D. Belin, M. J. Carson and J. Beckwith, 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177 4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann, G., F. Gotz and W. Lubitz, 1993. Expression of bacteriophage φX174 lysis gene E in Staphylococcus carnosus TM300. FEMS Microbiol. Lett. 108 139–143. [DOI] [PubMed] [Google Scholar]
- Hayashi, M., F. K. Fujimura and M. Hayashi, 1976. Mapping of in vivo messenger RNAs for bacteriophage phiX-174. Proc. Natl. Acad. Sci. USA 73 3519–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich, B., W. Lubitz and R. Plapp, 1982. Lysis of Escherichia coli by induction of cloned φX174 genes. Mol. Gen. Genet. 185 493–497. [DOI] [PubMed] [Google Scholar]
- Hutchison, C. A., III, S. Phillips, M. H. Edgell, S. Gillam, P. Jahnke et al., 1978. Mutagenesis at a specific position in a DNA sequence. J. Biol. Chem. 253 6551–6560. [PubMed] [Google Scholar]
- Karnik, S., and M. Billeter, 1983. The lysis function of RNA bacteriophage Qβ is mediated by the maturation (A2) protein. EMBO J. 2 1521–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolisnychenko, V., G. Plunkett, III, C. D. Herring, T. Feher, J. Posfai et al., 2002. Engineering a reduced Escherichia coli genome. Genome Res. 12 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman, M. A., 1994. A family of UDP-GlcNAc/MurNAc: polyisoprenol-P GlcNAc/MurNAc-1-P transferases. Glycobiology 4 768–771. [DOI] [PubMed] [Google Scholar]
- Link, A. J., D. Phillips and G. M. Church, 1997. Methods for generating precise deletions and insertions in the genome of wild-type Escherichia coli: application to open reading frame characterization. J. Bacteriol. 179 6228–6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd, A. J., P. E. Brandish, A. M. Gilbey and T. D. Bugg, 2004. Phospho-N-acetyl-muramyl-pentapeptide translocase from Escherichia coli: catalytic role of conserved aspartic acid residues. J. Bacteriol. 186 1747–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopilato, J., S. Bortner and J. Beckwith, 1986. Mutation in a new chromosonal gene of Escherichia coli K-12, pcnB, reduce plasmid copy number of pBR322 and its derivatives. Mol. Gen. Genet. 205 285–290. [DOI] [PubMed] [Google Scholar]
- Maratea, D., K. Young and R. Young, 1985. Deletion and fusion analysis of the φX174 lysis gene E. Gene 40 39–46. [DOI] [PubMed] [Google Scholar]
- Mendel, S., J. M. Holbourn, J. A. Schouten and T. D. Bugg, 2006. Interaction of the transmembrane domain of lysis protein E from bacteriophage φX174 with bacterial translocase MraY and peptidyl-prolyl isomerase SlyD. Microbiology 152 2959–2967. [DOI] [PubMed] [Google Scholar]
- Miller, J. H., 1972. a Experiment 16: 2-aminopurine and nitrous acid mutagenesis, pp. 135–139 in Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Miller, J. H., 1972. b Experiments in Molecular Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Pace, H. C., M. A. Kercher, P. Lu, P. Markiewicz, J. H. Miller et al., 1997. Lac repressor genetic map in real space. Trends Biochem. Sci. 22 334–339. [DOI] [PubMed] [Google Scholar]
- Ramanculov, E. R., and R. Young, 2001. Functional analysis of the T4 t holin in a lambda context. Mol. Genet. Genomics 265 345–353. [DOI] [PubMed] [Google Scholar]
- Roof, W. D., S. M. Horne, K. D. Young and R. Young, 1994. slyD, a host gene required for φX174 lysis, is related to the FK506-binding protein family of peptidyl-prolyl cis-trans-isomerases. J. Biol. Chem. 269 2902–2910. [PubMed] [Google Scholar]
- Sanger, F., G. M. Air, B. G. Barrell, N. L. Brown, A. R. Coulson et al., 1977. Nucleotide sequence of bacteriophage φX174 DNA. Nature 265 687. [DOI] [PubMed] [Google Scholar]
- Shen, H., and J. J. Chou, 2008. MemBrain: improving the accuracy of predicting transmembrane helices. PLoS ONE 3 e2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran, T. A. T., D. K. Struck and R. Young, 2005. Periplasmic domains define holin-antiholin interactions in T4 lysis inhibition. J. Bacteriol. 187 6631–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter, R. B., and L. Gold, 1983. Overproduction of bacteriophage Qβ maturation (A2) protein leads to cell lysis. Cell 33 877–885. [DOI] [PubMed] [Google Scholar]
- Young, K. D., and R. Young, 1982. Lytic action of cloned φX174 gene E. J. Virol. 44 993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, R., I. N. Wang and R. Calendar, 2006. Phage lysis, pp. 104–126 in The Bacteriophages. Oxford University Press, Oxford.
- Zheng, Y., D. K. Struck, C. A. Dankenbring and R. Young, 2008. Evolutionary dominance of holin lysis systems derives from superior genetic malleability. Microbiology 154 1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]