Abstract
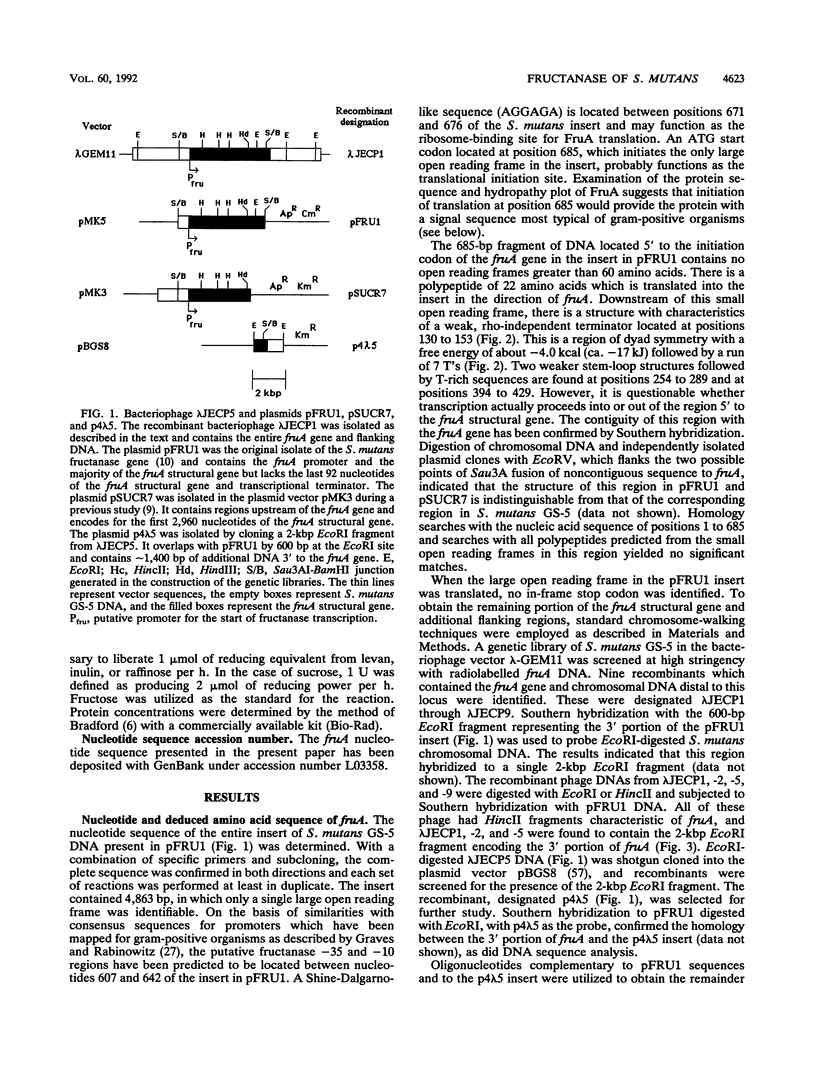
The complete nucleotide sequence (5,010 bp) of the fructanase gene (fruA) and flanking regions of the chromosome of Streptococcus mutans GS-5 was determined. The fruA gene appears to be the sole transcript arising from a proximal promoter. The presumed precursor of the secreted FruA protein consists of 1,423 amino acids, and it has an M(r) of 158,656 and a pI of 4.82. The N terminus of FruA has characteristics in common with signal peptides of gram-positive organisms. The C terminus consists of a serine- and threonine-rich region, followed by the peptide LPDTGD, 4 charged amino acids, 21 amino acids with a strongly hydrophobic character, and a charged pentapeptide tail, which are proposed to correspond to the wall-spanning region, the LPXTGX consensus sequence, and the membrane-spanning domains of surface-associated proteins of gram-positive cocci. The FruA protein has significant homology with the Bacillus subtilis levanase (SacC), the Bacteroides fragilis levanase (ScrL), yeast invertases, and a number of other beta-fructosidases but not with fructosyltransferase, glucosyltransferases, or glucan-binding proteins of oral streptococci. Genes with homology to fruA were detected in S. mutans serotype c, e, and f strains, Streptococcus rattus, Streptococcus salivarius, and Streptococcus sanguis. A deletion derivative of FruA lacking the C-terminal 437 amino acids was still functional and could hydrolyze beta-(2,6)- and beta-(2,1)-linked sugars, but with altered preference for substrates. The data begin to define functional domains of the FruA protein and potential regulatory sites for induction, repression, growth rate control, and posttranslational localization of this multifunctional enzyme.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender G. R., Sutton S. V., Marquis R. E. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986 Aug;53(2):331–338. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkhed D., Rosell K. G., Granath K. Structure of extracellular water-soluble polysaccharides synthesized from sucrose by oral strains of Streptococcus mutans, Streptococcus salivarius, Streptococcus sanguis and Actinomyces viscosus. Arch Oral Biol. 1979;24(1):53–61. doi: 10.1016/0003-9969(79)90175-4. [DOI] [PubMed] [Google Scholar]
- Bowden G. H., Hamilton I. R. Environmental pH as a factor in the competition between strains of the oral streptococci Streptococcus mutans, S. sanguis, and "S. mitior" growing in continuous culture. Can J Microbiol. 1987 Sep;33(9):824–827. doi: 10.1139/m87-143. [DOI] [PubMed] [Google Scholar]
- Bowden G. H. Microbiology of root surface caries in humans. J Dent Res. 1990 May;69(5):1205–1210. doi: 10.1177/00220345900690051701. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burne R. A., Rubinfeld B., Bowen W. H., Yasbin R. E. Tight genetic linkage of a glucosyltransferase and dextranase of Streptococcus mutans GS-5. J Dent Res. 1986 Dec;65(12):1392–1401. doi: 10.1177/00220345860650120301. [DOI] [PubMed] [Google Scholar]
- Burne R. A., Schilling K., Bowen W. H., Yasbin R. E. Expression, purification, and characterization of an exo-beta-D-fructosidase of Streptococcus mutans. J Bacteriol. 1987 Oct;169(10):4507–4517. doi: 10.1128/jb.169.10.4507-4517.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter-Muenchau P., Wolf R. E., Jr Growth-rate-dependent regulation of 6-phosphogluconate dehydrogenase level mediated by an anti-Shine-Dalgarno sequence located within the Escherichia coli gnd structural gene. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1138–1142. doi: 10.1073/pnas.86.4.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassy B. M. A gentle method for the lysis of oral streptococci. Biochem Biophys Res Commun. 1976 Jan 26;68(2):603–608. doi: 10.1016/0006-291x(76)91188-8. [DOI] [PubMed] [Google Scholar]
- Chisholm D. A convenient moderate-scale procedure for obtaining DNA from bacteriophage lambda. Biotechniques. 1989 Jan;7(1):21–23. [PubMed] [Google Scholar]
- DaCosta T., Gibbons R. J. Hydrolysis of levan by human plaque streptococci. Arch Oral Biol. 1968 Jun;13(6):609–617. doi: 10.1016/0003-9969(68)90139-8. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Kato K., Kotani S., Misaki A. Structural differences in fructans elaborated by streptococcus mutans and Strep. salivarius. J Biochem. 1975 Nov;78(5):879–887. doi: 10.1093/oxfordjournals.jbchem.a130993. [DOI] [PubMed] [Google Scholar]
- Ehrlich J., Stivala S. S., Bahary W. S., Garg S. K., Long L. W., Newbrun E. Levans: I. Fractionation, solution viscosity, and chemical analysis of levan produced by Streptococcus salivarius. J Dent Res. 1975 Mar-Apr;54(2):290–297. [PubMed] [Google Scholar]
- Ferretti J. J., Russell R. R., Dao M. L. Sequence analysis of the wall-associated protein precursor of Streptococcus mutans antigen A. Mol Microbiol. 1989 Apr;3(4):469–478. doi: 10.1111/j.1365-2958.1989.tb00193.x. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Pancholi V., Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol Microbiol. 1990 Sep;4(9):1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Fouet A., Arnaud M., Klier A., Rapoport G. Bacillus subtilis sucrose-specific enzyme II of the phosphotransferase system: expression in Escherichia coli and homology to enzymes II from enteric bacteria. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8773–8777. doi: 10.1073/pnas.84.24.8773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour M. N., Whittam T. S., Kilian M., Selander R. K. Genetic relationships among the oral streptococci. J Bacteriol. 1987 Nov;169(11):5247–5257. doi: 10.1128/jb.169.11.5247-5257.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N., Vapnek D. A simple method of preparing large amounts of phiX174 RF 1 supercoiled DNA. Biochim Biophys Acta. 1973 Apr 11;299(4):516–520. doi: 10.1016/0005-2787(73)90223-2. [DOI] [PubMed] [Google Scholar]
- Gold W., Preston F. B., Lache M. C., Blechman H. Production of levan and dextran in plaque in vivo. J Dent Res. 1974 Mar-Apr;53(2):442–446. doi: 10.1177/00220345740530024401. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Rabinowitz J. C. In vivo and in vitro transcription of the Clostridium pasteurianum ferredoxin gene. Evidence for "extended" promoter elements in gram-positive organisms. J Biol Chem. 1986 Aug 25;261(24):11409–11415. [PubMed] [Google Scholar]
- Guggenheim B., Burckhardt J. J. Isolation and properties of a dextranase from streptococcus mutans OMZ 176. Helv Odontol Acta. 1974 Oct;18(2):101–113. [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy L., Jacques N. A., Forester H., Campbell L. K., Knox K. W., Wicken A. J. Effect of fructose and other carbohydrates on the surface properties, lipoteichoic acid production, and extracellular proteins of Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1981 Jan;31(1):78–87. doi: 10.1128/iai.31.1.78-87.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M., Iwami Y., Yamada T., Araya S. Levan synthesis and accumulation by human dental plaque. Arch Oral Biol. 1970 Jun;15(6):563–567. doi: 10.1016/0003-9969(70)90111-1. [DOI] [PubMed] [Google Scholar]
- Jacques N. J., Morrey-Jones J. G., Walker G. J. Inducible and constitutive formation of fructanase in batch and continuous cultures of Streptococcus mutans. J Gen Microbiol. 1985 Jul;131(7):1625–1633. doi: 10.1099/00221287-131-7-1625. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Recent advances in defining the cariogenicity of mutans streptococci: molecular genetic approaches. Eur J Epidemiol. 1987 Sep;3(3):257–260. doi: 10.1007/BF00149733. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- LUCHSINGER W. W., CORNESKY R. A. Reducing power by the dinitrosalicylic acid method. Anal Biochem. 1962 Oct;4:346–347. doi: 10.1016/0003-2697(62)90098-2. [DOI] [PubMed] [Google Scholar]
- Manly R. S., Richardson D. T. Metabolism of levan by oral samples. J Dent Res. 1968 Nov-Dec;47(6):1080–1086. doi: 10.1177/00220345680470061301. [DOI] [PubMed] [Google Scholar]
- Marshall K., Weigel H. Evidence of multiple branching in the levan elaborated by Streptococcus salivarius strain 51. Carbohydr Res. 1980 Aug 15;83(2):321–326. doi: 10.1016/s0008-6215(00)84544-9. [DOI] [PubMed] [Google Scholar]
- Martin-Verstraete I., Débarbouillé M., Klier A., Rapoport G. Levanase operon of Bacillus subtilis includes a fructose-specific phosphotransferase system regulating the expression of the operon. J Mol Biol. 1990 Aug 5;214(3):657–671. doi: 10.1016/0022-2836(90)90284-S. [DOI] [PubMed] [Google Scholar]
- Martin I., Débarbouillé M., Ferrari E., Klier A., Rapoport G. Characterization of the levanase gene of Bacillus subtilis which shows homology to yeast invertase. Mol Gen Genet. 1987 Jun;208(1-2):177–184. doi: 10.1007/BF00330439. [DOI] [PubMed] [Google Scholar]
- McDermid A. S., McKee A. S., Ellwood D. C., Marsh P. D. The effect of lowering the pH on the composition and metabolism of a community of nine oral bacteria grown in a chemostat. J Gen Microbiol. 1986 May;132(5):1205–1214. doi: 10.1099/00221287-132-5-1205. [DOI] [PubMed] [Google Scholar]
- Miller C. H., Somers P. J. Degradation of levan by Actinomyces viscosus. Infect Immun. 1978 Oct;22(1):266–274. doi: 10.1128/iai.22.1.266-274.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K., Takano T., Sohma A., Yamane K. Secretion activities of Bacillus subtilis alpha-amylase signal peptides of different lengths in Escherichia coli cells. Biochem Biophys Res Commun. 1986 Jan 29;134(2):624–631. doi: 10.1016/s0006-291x(86)80465-x. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. A., Maley F. Identification of an active-site residue in yeast invertase by affinity labeling and site-directed mutagenesis. J Biol Chem. 1990 Jul 5;265(19):10817–10820. [PubMed] [Google Scholar]
- Ritz H. L. Microbial population shifts in developing human dental plaque. Arch Oral Biol. 1967 Dec;12(12):1561–1568. doi: 10.1016/0003-9969(67)90190-2. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling K. M., Bowen W. H. Glucans synthesized in situ in experimental salivary pellicle function as specific binding sites for Streptococcus mutans. Infect Immun. 1992 Jan;60(1):284–295. doi: 10.1128/iai.60.1.284-295.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneewind O., Model P., Fischetti V. A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992 Jul 24;70(2):267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- Shimamura A., Tsuboi K., Nagase T., Ito M., Tsumori H., Mukasa H. Structural determination of D-fructans from Streptococcus mutans, serotype b, c, e, and f strains, by 13C-n.m.r. spectroscopy. Carbohydr Res. 1987 Jul 15;165(1):150–154. doi: 10.1016/0008-6215(87)80091-5. [DOI] [PubMed] [Google Scholar]
- Shiroza T., Kuramitsu H. K. Sequence analysis of the Streptococcus mutans fructosyltransferase gene and flanking regions. J Bacteriol. 1988 Feb;170(2):810–816. doi: 10.1128/jb.170.2.810-816.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms P. J., Boyko W. J., Edwards J. R. The structural analysis of a levan produced by Streptococcus salivarius SS2. Carbohydr Res. 1990 Dec 15;208:193–198. doi: 10.1016/0008-6215(90)80099-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Hedge P. J., te Heesen S., Edelman A., Broome-Smith J. K. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene. 1986;41(2-3):337–342. doi: 10.1016/0378-1119(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Mizuno F., Takamori K. Purification and preliminary characterization of exo-beta-D-fructosidase in Streptococcus salivarius KTA-19. Infect Immun. 1985 Jan;47(1):271–276. doi: 10.1128/iai.47.1.271-276.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend-Lawman P., Bleiweis A. S. Multilevel control of extracellular sucrose metabolism in Streptococcus salivarius by sucrose. J Gen Microbiol. 1991 Jan;137(1):5–13. doi: 10.1099/00221287-137-1-5. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Brown R. A., Taylor C. Activity of Streptococcus mutans alpha-D-glucosyltransferases released under various growth conditions. J Dent Res. 1984 Mar;63(3):397–400. doi: 10.1177/00220345840630030801. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Hare M. D., Morrey-Jones J. G. Activity of fructanase in batch cultures of oral streptococci. Carbohydr Res. 1983 Feb 16;113(1):101–112. doi: 10.1016/0008-6215(83)88222-6. [DOI] [PubMed] [Google Scholar]
- Warner T. N., Miller C. H. Cell-associated levan of Actinomyces viscosus. Infect Immun. 1978 Feb;19(2):711–719. doi: 10.1128/iai.19.2.711-719.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler D. L., Penders J. E., Bowen W. H., Burne R. A. Characteristics and cariogenicity of a fructanase-defective Streptococcus mutants strain. Infect Immun. 1992 Sep;60(9):3673–3681. doi: 10.1128/iai.60.9.3673-3681.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M. The amount, distribution and metabolism of soluble polysaccharides in human dental plaque. Arch Oral Biol. 1967 Jul;12(7):849–858. doi: 10.1016/0003-9969(67)90107-0. [DOI] [PubMed] [Google Scholar]
- van Houte J., Jansen H. M. Levan degradation by streptococci isolated from human dental plaque. Arch Oral Biol. 1968 Jul;13(7):827–830. doi: 10.1016/0003-9969(68)90102-7. [DOI] [PubMed] [Google Scholar]
- von Heijne G., Abrahmsén L. Species-specific variation in signal peptide design. Implications for protein secretion in foreign hosts. FEBS Lett. 1989 Feb 27;244(2):439–446. doi: 10.1016/0014-5793(89)80579-4. [DOI] [PubMed] [Google Scholar]