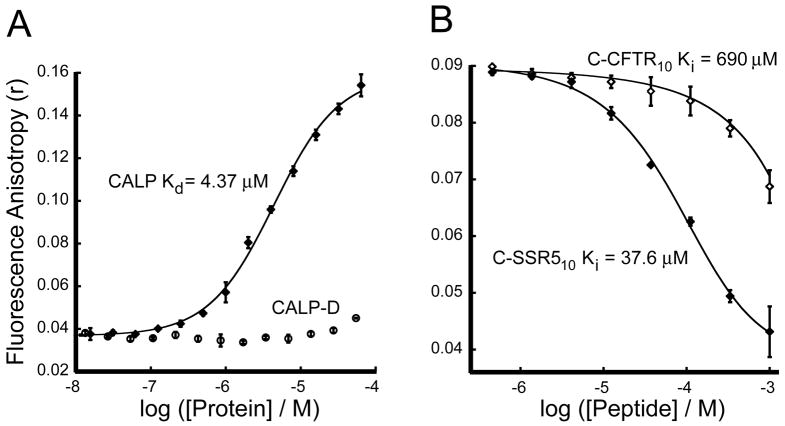
Figure 7. CALP binds the C-terminus of CFTR with very low affinity.
(A) Direct binding analysis of a high affinity reporter: Fluorescence anisotropy was determined following titration of 30 nM F*C-SSR510 reporter peptide with increasing concentrations of CALP (diamonds) or CALP-D mutant (circles). Curve fitting (solid line) yields an estimate for Kd = 4.37 ± 0.31 μM. (B) Competition binding analysis: Fluorescence anisotropy data were obtained for 30 nM F*C-SSR510 and 5 μM CALP (~ 1 × Kd) incubated with increasing concentrations of either unlabeled C-SSR510 (filled diamonds, Ki = 37.6 ± 4.0 μM) or unlabeled C-CFTR10 (open diamonds, Ki = 690 ± 120 μM). Fits are shown as solid lines. Values shown are mean ± SD, n=3.