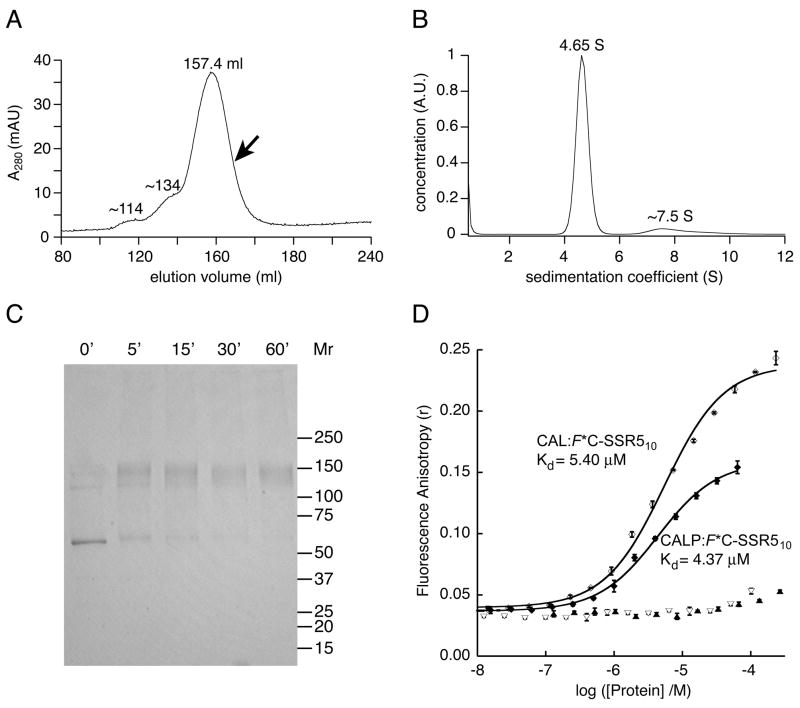
Figure 8. The CAL PDZ domain recapitulates the affinity of full-length dimeric CAL for CFTR.
(A) Size-exclusion chromatography (SEC) of H10-CAL reveals a dominant peak at Ve=157.4 ml. Two additional minor peaks are seen as well, with smaller elution volumes. The arrow indicates the position of the fraction collected for velocity sedimentation experiments. Standard proteins were also subjected to SEC and used to calibrate the diffusion coefficient (D20,w) of H10-CAL. (B) The sedimentation coefficient concentration distribution c(s) (solid line) was determined for SEC-purified CAL using velocity sedimentation analysis. The major peak is shown at 4.65 S, together with minor faster-sedimenting components exhibiting a mode of ~7.5 S and a mean of ~9.1 S. (C) A Coomassie-stained SDS-PAGE gel is shown of H10-CAL samples cross-linked with BS3. The reaction was quenched with ethanolamine at the time indicated, revealing the time-dependent formation of a covalent dimer that was essentially complete at 60 min. (D) Fluorescence anisotropy data were obtained in the presence of increasing concentrations of CAL for 30 nM F*C-SSR510 (open diamonds) or 30 nM F*-CFTR10 (open triangles). The solid line represents a fit of the F*C-SSR510 data to a single-site binding model (Kd = 5.40 ± 0.47 μM). The interaction with F*-CFTR10 was too weak to be fit. For comparison, equivalent titrations are shown for the CALP protein (closed diamonds and closed triangles, respectively). Values shown are mean ± SD, n=3.