Fig. 3. Effect of OPLS on Stability of rFVIII.
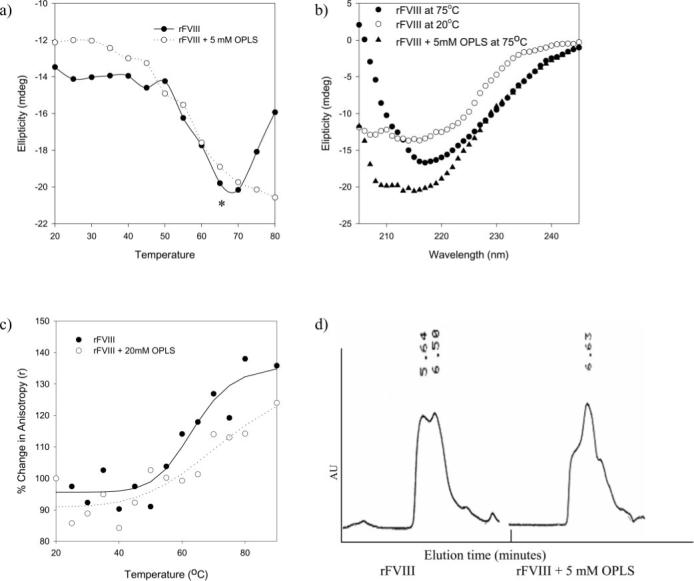
a, temperature-dependant ellipticity changes of rFVIII in the presence (○) and in the absence (●) of OPLS monitored at 215 nm. Secondary structural transition of rFVIII was monitored over the temperature range of 20−80 °C at a heating rate of 60 °C/h. The concentration of the protein used was ∼25 μg/100 μl, and the path length of the quartz cuvette was 0.1 cm. b, CD spectra of rFVIII in the presence and in the absence of OPLS acquired at various temperatures. Open symbols, 20 °C; closed symbols, 75 °C. c, temperature-dependent changes in the steady state fluorescence anisotropy of rFVIII in the presence (○) and in the absence (●) of 20 mm OPLS. Changes in anisotropy were monitored over the temperature range of 20−80 °C at a heating rate of 60 °C/h. The protein concentration was ∼5 μg/ml. The excitation wavelength was set at 280 nm, and the emission was monitored at 335 nm. d, a representative size exclusion chromatography profile of rFVIII obtained at 55 °C in the presence and in the absence of OPLS. rFVIII was subjected to thermal stress over the temperature range of 25−75 °C at 60 °C/h, and elution was monitored using a fluorescence detector with excitation and emission wavelengths set at 285 and 335 nm, respectively. The elution time is shown in minutes. Gel filtration was carried out under isocratic conditions at a flow rate of 0.4 ml/min. Samples were cooled down and stored for not more than 60 min at 4 °C prior to analysis. The column temperature was maintained at 20 °C. AU, arbitrary units.
