Table 2.
Substrate Scopea
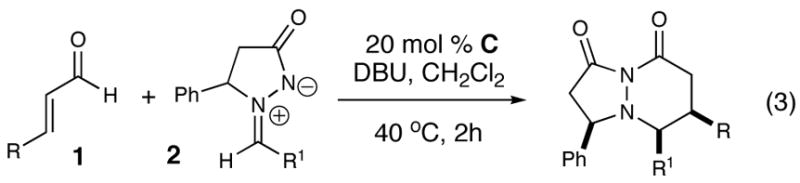 | |||||
|---|---|---|---|---|---|
| entry | R | R1 | product | yield (%)b | d.r.c |
| 1 | Ph (1a) | Ph (2a) | 4 | 79 | >20:1 |
| 2 | 4-OMePh(1b) | Ph (2a) | 5 | 76 | >20:1 |
| 3 | 3-OMePh(1c) | Ph (2a) | 6 | 79 | >20:1 |
| 4 | 2-OMePh (1d) | Ph (2a) | 7 | 94 | >20:1 |
| 5 | 2-naphthyl (1e) | Ph (2a) | 8 | 77 | >20:1 |
| 6 | CH2CH2CH3(1f) | Ph (2a) | 9 | 67 | >20:1 |
| 7 | HC=CHCH3(1g) | Ph (2a) | 10 | 51 | >20:1 |
| 8 | 4-CIPh(1h) | Ph (2a) | 11 | 0 | - |
| 9 | Ph (1a) | 4-BrPh (2b) | 12 | 87 | >20:1 |
| 10 | Ph (1a) | 4-FPh (2c) | 13 | 82 | >20:1 |
| 11 | Ph (1a) | 3-CF3Ph (2d) | 14 | 93 | >20:1 |
| 12 | Ph (1a) | 3-BrPh (2e) | 15 | 78 | >20:1 |
| 13 | Ph (1a) | 3-CH3Ph (2f) | 16 | 76 | >20:1 |
| 14 | Ph (1a) | 3-OMePh (2g) | 17 | 67 | >20:1 |
| 15 | Ph (1a) | cyclohexyl (2h) | 18 | 0 | - |
2 equiv. 1 and 1 equiv. 2,0.25 M.
Isolated yield after purification.
Determined by 500 MHz
1H NMR spectroscopy. Relative stereochemistry of 16 determined by X-ray crystallography and further assigned by analogy. See Supporting Information for details.
