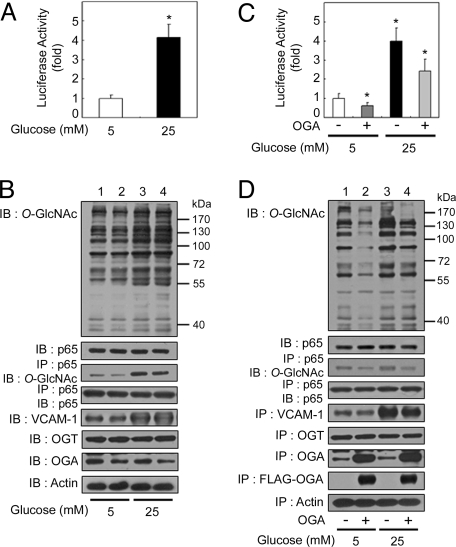
Fig. 1.
OGA overexpression-mediated decrease in O-GlcNAcylation suppresses hyperglycemia-induced NFκB activation. (A) Rat VSMCs were transfected with the κB-luciferase reporter gene plasmid and then incubated for 24 h under normal (5 mM) or high-glucose (25 mM) conditions. The luciferase activity was measured and normalized to β-galactosidase activity from a cotransfected control plasmid. The data shown represent the mean ± SD (n = 3); *, P < 0.01 by Student's t test. (B) VSMCs were exposed to normal-glucose (lanes 1 and 2) or high-glucose (lanes 3 and 4) levels for 24 h. The levels of O-GlcNAc, NFκB p65, VCAM-1, OGT, and OGA were determined in total cell lysates by immunoblotting (IB; 1st, 2nd, and 5th–7th panels, respectively). NFκB p65 immunoprecipitates (IP) obtained from cellular extracts were analyzed by immunoblotting for O-GlcNAc and NFκB p65 (3rd and 4th panels, respectively). Actin was included as a loading control (8th panel). (C) VSMCs were cotransfected with the κB-luciferase reporter gene plasmid and the plasmid expressing FLAG-tagged human OGA, and incubated for 24 h under normal- or high-glucose conditions. The luciferase activity was measured and normalized to β-galactosidase activity, and the data shown represent the mean ± SD (n = 3); *, P < 0.01 by Student's t test. (D) VSMCs transfected with empty vector (lanes 1 and 3) or that encoding FLAG-tagged OGA (lanes 2 and 4) were exposed to normal-glucose (lanes 1 and 2) or high-glucose (lanes 3 and 4) levels for 24 h. The levels of O-GlcNAc, NFκB p65, VCAM-1, OGT, OGA, and FLAG-tagged OGA were determined in total cell lysates by immunoblotting (1st, 2nd, and 5th–8th panels, respectively). NFκB p65 immunoprecipitates obtained from the cellular extracts were analyzed by immunoblotting for O-GlcNAc and NFκB p65 (3rd and 4th panels, respectively). Actin was included as a loading control (9th panel).