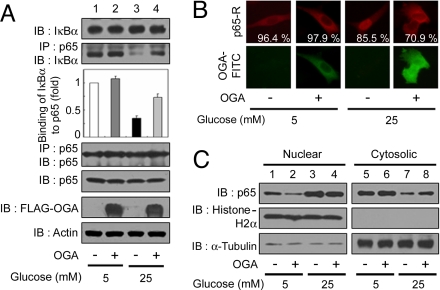
Fig. 2.
OGA overexpression inhibits the reduction in NFκB p65–IκBα interactions and nuclear translocation of NFκB p65 under high-glucose conditions. VSMCs were transfected with either vector or a plasmid expressing FLAG-tagged OGA and exposed to high glucose for 24 h. (A) Immunoblotting (IB) for NFκB p65, IκBα, and FLAG-tagged OGA was performed with an anti-NFκB p65 antibody, an anti-IκBα antibody, and an anti-FLAG antibody (1st, 5th, and 6th panels, respectively). NFκB p65 immunoprecipitates (IP) were analyzed for IκBα and NFκB p65 expression by using immunoblotting with the corresponding antibodies (2nd and 4th panels, respectively). Actin was used as a loading control (7th panel). (B) (Upper) VSMCs were immunostained by using antibodies against NFκB p65 followed by anti-rabbit Ig covalently conjugated to rhodamine (R) to determine the subcellular localization. (Lower) OGA overexpression was detected with an anti-FLAG antibody conjugated to FITC. The percentages of cells showing NFκB p65 localization are derived from at least 150 transfected cells with FLAG-OGA in microscopic fields. (C) Immunoblotting of nuclear or cytosolic extracts of VSMCs by using antibodies against NFκB p65 was performed to determine NFκB p65 nuclear translocation (1st row). The purity of the nuclear and cytosolic extracts was determined by immunoblotting of histone H2A (nuclear fractions, 2nd row) and α-tubulin (cytosolic fractions, 3rd row).