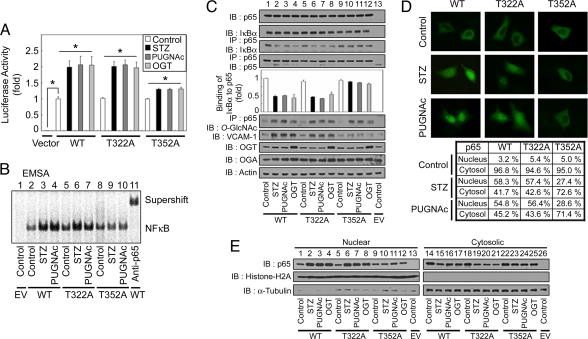
Fig. 5.
Mutation of the NFκB p65 O-GlcNAcylation site at Thr-352 abrogates O-GlcNAc-induced NFκB transcriptional activation. (A) NFκB p65 KO MEFs were transfected with empty vector, the κB-luciferase reporter gene plasmid, the plasmid encoding wild-type FLAG-tagged IκBα, and the WT or mutant NFκB p65 (T322A and T352A) His-tagged expression vectors. The transfected cells were then incubated for 12 h in the absence (control) or presence of STZ (2 mM), PUGNAc (100 μM), or transfected with the plasmid expressing FLAG-tagged OGT as indicated, and the luciferase activity was measured and normalized to β-galactosidase activity. The data shown represent the mean ± SD (n = 3); *, P < 0.01 by Student's t test. (B) NFκB p65 KO MEFs were cotransfected with empty vector (EV) or plasmids encoding FLAG-tagged IκBα, and plasmids encoding the WT or mutant His-tagged NFκB p65 proteins were incubated for 12 h in the absence (control) or presence of STZ or PUGNAc. Nuclear NFκB DNA-binding affinity was analyzed by EMSA using 32P-radiolabeled κB-enhancer probes. The supershift assay was examined with antibodies against NFκB p65 (rabbit polyclonal). (C) Plasmids encoding His-NFκB p65 (WT or mutants) and FLAG-IκBα were cotransfected into NFκB p65 KO MEFs. After a 12-h incubation in the absence (control) or presence of STZ, PUGNAc, or after transfection with the plasmid expressing FLAG-tagged OGT, immunoblotting for NFκB p65, IκBα, VCAM-1, OGA, and OGA was performed by using the corresponding antibodies (1st, 2nd, and 7th–9th panels, respectively). NFκB p65 WT and mutant proteins were also immunoprecipitated from cell lysates, and the immunoprecipitates were analyzed for IκBα, NFκB p65, and O-GlcNAc by using immunoblotting (3rd, 4th, and 6th panels, respectively). Actin was used as a loading control (10th panel). (D) Plasmids encoding His-NFκB p65 (WT or mutants) and FLAG-IκBα were cotransfected into NFκB p65 KO MEFs and incubated for 12 h in the absence (control) or presence of STZ or PUGNAc. Immunostaining for NFκB p65 was performed to determine the subcellular localization of His-NFκB p65 WT and mutants. The table indicates percentage localization in each compartment, and the percentages are derived from at least 200 transfected cells in microscopic fields. (E) NFκB p65 KO MEFs were cotransfected with empty vector (EV) or plasmids containing His-NFκB p65 (WT or mutants) and FLAG-tagged IκBα, and incubated for 12 h in the presence or absence of STZ, PUGNAc, or transfected with a plasmid expressing FLAG-tagged OGT. Immunoblotting of nuclear or cytosolic extracts by using antibodies against NFκB p65 was performed to determine NFκB p65 nuclear translocation (1st row). The purity of the nuclear and cytosolic extracts was determined by immunoblotting of histone H2A (nuclear fractions, 2nd row) and α-tubulin (cytosolic fractions, 3rd row).