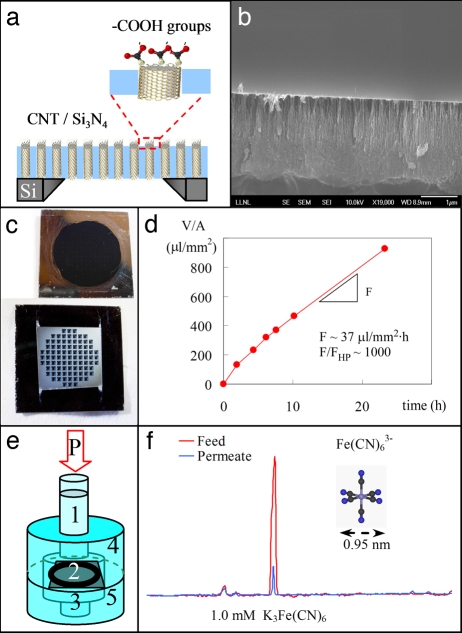
Fig. 1.
CNT/silicon nitride membrane platform for ultrafast nanofiltration of electrolytes. (a) Cross-section schematic of a CNT membrane representing the silicon support chip, the aligned DWNTs, the filling silicon nitride matrix, and the CNT tips functionalized with carboxylic groups. (b) Cross-section SEM image of the CNT/silicon nitride composite showing the gap-free coating of silicon nitride. (c) Photographs of the membrane sides exposed to the feed (Upper) and to the permeate (Lower). (d) Time variation of permeate volume per unit area of freestanding membrane during the filtration of 0.6 mM K3Fe(CN)6 solution. The resulting permeation flux, F, is ≈1,000 larger than the calculated value with the Hagen–Poiseuille equation, FHP. (e) Schematic of the nanofiltration cell showing the column of feed solution (1) pressurized at P = 0.69 bar, the CNT membrane (2), the permeate solution (3), and feed (4) and permeate (5) chambers. (f) Capillary electrophoresis chromatogram for feed (red) and permeate (blue) showing a 91% exclusion of the ferricyanide anion after nanofiltration of a 1.0 mM K3Fe(CN)6 solution.