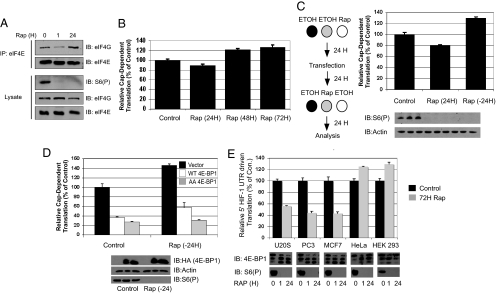
Fig. 4.
Rapamycin-induced 4E-BP1 phosphorylation stimulates cap-dependent translation and regulates cell-specific inhibition of translation. (A) The interaction between eIF4E and eIF4G was measured in coimmunoprecipitation experiments in HEK293 cells treated with rapamycin for 1 or 24 h. (B) In vivo cap-dependent translational assays were performed except rapamycin was added 3 h after transfection for 24, 48, or 72 h. (C) Phospho-S6 and actin were blotted for normalization. (D) The experiments were completed in the same way as in C, except WT 4E-BP1, AA 4E-BP1, or a control vector was cotransfected. Phospho-S6, actin, and HA (4E-BP1) blots are shown. Black, control vector; white, WT 4E-BP1; gray, AA 4E-BP1. (E) Different cell lines that differentially exhibit rapamycin-induced 4E-BP1 hyperphosphorylation were transfected with the HIF-1α 5′ UTR-driven translational vector and were treated with rapamycin or ethanol 3 h after transfection for 72 h. Black, control; gray, rapamycin (20 nM). The 4E-BP1 and S6(P) Western blots are shown in conjunction with the translational data.