Abstract
Aim
To assess the relationship between protein and messenger RNA (mRNA) levels of vascular endothelial growth factor (VEGF) and subcellular localization of nuclear factor-kappa B (NF-κB), proliferation rate of tumor cells, and clinicopathological characteristics of renal cell tumors.
Methods
We analyzed 31 one renal cell tumors – 22 clear cell renal cell carcinomas (CCRCC) and 9 other histologic types (non-CCRCC). VEGF expression and subcellular localization of p65 member of NF-κB and Ki67 were immunohistochemically evaluated for the proliferation rate of tumor cells. Expression of VEGF mRNA was assessed using quantitative real-time polymerase chain reaction after total RNA extraction from snap-frozen tumor tissue samples.
Results
Cytoplasmic localization of VEGF protein in renal cell tumors showed a perimembranous and diffuse pattern, the former being more evident in CCRCC (27.1 ± 18.9 vs 3.3 ± 10 % tumors, P = 0.001) and the latter in non-CCRCC type (71.7 ± 23.2 vs 31.1 ± 22.1 % tumors, P < 0.001). Heterogeneity in VEGF gene expression was more pronounced in CCRCC type than in non-CCRCC type (P = 0.004). In addition, perimembranous VEGF pattern was associated with higher VEGF mRNA levels (P = 0.006) and diffuse VEGF pattern with lower VEGF mRNA levels (P < 0.001). Nuclear and cytoplasmic staining of NF-κB/p65 was observed in the majority of tumor cells. A significant association was recorded between cytoplasmic NK-κB/65 staining and VEGF staining of diffuse pattern (P = 0.026). Association between NF-κB/65 and proliferation rate of tumor cells was significant for cytoplasmic staining (P = 0.039) but not for nuclear NFkB/p65 staining (P = 0.099).
Conclusion
Higher but inhomogeneous expression of VEGF in tumor cells, especially in CCRCCs, is associated with NF-κB/65 activity. This indicates that both VEGF and NF-κB/65 may be important in renal carcinogenesis, representing a possible molecular target in the treatment of renal cell carcinoma.
Renal cell carcinoma is a malignancy with a variable clinical course, which is partly attributable to different genetic structure of different renal cell carcinoma types (1). The majority of clear cell renal cell carcinoma (CCRCC) types are associated with the loss of heterozygosity in several regions along the short arm of chromosome 3, including the regions where von Hippel-Lindau, FHIT, RASSF1A, and DRR1 genes are located. The loss of function of von Hippel-Lindau gene leads to aberrant activation of hypoxic response in terms of up-regulation of angiogenic factors and consequent neovascularization (2).
Angiogenesis is an important process for tumor progression and metastatic spread. One of the major factors that regulate this process is vascular endothelial growth factor (VEGF) (3). Angiogenic factors are newly synthesized by the mechanism of inducible transcriptional initiation of their genes. This phenomenon is governed by transcription factors, which bind to the regulatory regions of genes.
The nuclear factor-kappa B (NF-κB) is a transcription factor that plays an important role in the control of growth, differentiation, and apoptosis. It consists of homodimers and heterodimers composed of several subunits as follows: NF-κB1 (p50/p105), NF-κB2 (p52/100), Rel A (p65), Rel B, and c-Rel proteins (4). The inactive form of NF-κB is localized in the cytoplasm and consists of the DNA-binding p50 and p65 subunits and an inhibitory subunit called IκB, which is bound to p65. IκB masks the nuclear localization sequence, and its release initiates the activation of NF-κB and its subsequent translocation to the nucleus, where it can bind to DNA target sites (5). Activation of NF-κB may be triggered by a variety of cytokines, growth factor receptors, and tyrosine kinase.
Activation of NF-κB results in the induction of a large number of genes involved in the regulation of a wide variety of biological responses, including anti-apoptotic genes, cell cycle-regulatory genes, genes encoding adhesion molecules, chemokines, inflammatory cytokines, genes involved in metastases, cyclooxygenase, and VEGF (6-10). There is also increasing evidence that NF-κB is implicated in oncogenesis (11).
The aim of this study was to assess protein and messenger RNA (mRNA) levels of VEGF and to compare their values with subcellular localization of NF-κB, proliferation rate of tumor cells, and clinicopathological characteristics of renal cell tumors.
Patients and methods
Clinicopathological data
We collected unfixed material from 70 consecutive nephrectomy specimens from patients surgically treated for renal cell neoplasm in the period from 2003 to 2004 at the Department of Urology, Rijeka University Hospital Center, Rijeka, Croatia. The final sample comprised 31 renal cell tumor and 22 non-tumor nephrectomy specimens with adequate mRNA for further real-time polymerase chain reaction (PCR). All samples were snap-frozen in liquid nitrogen, stored at -80°C, fixed in 4% buffered formalin, embedded in paraffin, and routinely stained with hematoxylin and eosin. Data on sex, age, tumor size, tumor-node-metastases stage, histologic subtype according to the World Health Organization classification (12), and nuclear grade according to the Fuhrman nuclear grading system (13) were obtained from patient medical records and files of the Department of Pathology, Rijeka University School of Medicine.
Immunohistochemistry
Tumor samples were immunohistologically analyzed in a Dako Autostainer Plus (DakoCytomation Colorado Inc, Fort Collins, CO, USA) according to the manufacturer’s protocol, using Envision peroxidase procedure (ChemMate TM Envision HRP detection Kit K5007, DakoCytomation, Glostrup, Denmark). Epitope retrieval was achieved by immersing slides in Tris-EDTA buffer (pH 9.0) and boiling water bath for 10 minutes. Slides were left to cool for 45 minutes and were preincubated with blocking solution, containing normal goat serum (DakoCytomation), for 30 minutes. VEGF expression was determined by mouse monoclonal antibody VEGF (C-1: sc-7269, dilution 1:100, overnight incubation at 4şC; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Subcellular localization of p65 member of NF-κB was determined with mouse monoclonal antibody NF-κB p65 (F-6: sc-8008; dilution 1:50, overnight incubation at 4°C; Santa Cruz Biotechnology). Proliferative activity was assessed by detecting Ki67 protein with monoclonal antibody (clone MIB-1, dilution 1:50; DakoCytomation).
EnVisionTM G/2 Doublestain System (K5361, DakoCytomation) was used for detection of Ki67 nuclear positivity with chromogen DAB and VEGF cytoplasmic positivity with Fast Red. Negative control slides were prepared by substituting Dako ChemMate antibody diluent for secondary antibody.
Evaluation of immunostaining
Immunohistochemical staining results were evaluated independently by two pathologists unaware of the clinicopathological data of the patients. No interobserver variability was found for the two observers.
The intensity of VEGF immunostaining was semiquantitatively assessed as follows: 0 – none, 1 – weak, 2 – moderate, and 3 – strong. The immunoreactive score was calculated by multiplying the intensity (I) with the percentage of positive tumor cells (VEGF Histo-score = [percentage of positive tumor cells × I1]+[percentage of positive tumor cells × I2]+[percentage of positive tumor cells × I3]). Furthermore, VEGF cytoplasmic expression was evaluated as the percentage of diffuse and perimembranous staining pattern in tumor cells. Smooth muscle cells in vascular walls served as internal control. For statistical analysis, we used only VEGF expression, determined as diffuse and perimembranous, since the analysis with VEGF H-score showed no significant results.
Nuclear and cytoplasmic NF-κB/p65 intensity of immunoreactivity was graded as follows: 0 – none, 1 – weak, 2 – moderate, and 3 – strong. The percentage of neoplastic cells with cytoplasmic staining was approximately determined in the entire tumor area, while the percentage of nuclear staining was determined by counting positive tumor nuclei in 500 tumor cells in the tumor areas with the highest density of positive cells, using ×60 power field and squared graticule (U-OCMSQ7/7, Olympus, Zagreb, Croatia). The immunoreactive score for cytoplasmic and nuclear staining of NF-κB/p65 (Histo-score) was calculated in the same way as the score for VEGF. Cytoplasmic staining of tumor and non-tumor cells served as internal positive control.
Ki67 index (percentage of positive cells) was determined by counting 500 tumor cells under ×400 magnification in the tumor areas with the highest proliferative activity. The counting was performed by ISSA 3.1 software (Vams, Zagreb, Croatia). A sample was considered positive if any nuclear staining was seen.
RNA isolation, reverse transcription PCR, and real-time PCR
Fresh tissue was homogenized using MagNA Lyser (Roche Applied Science, Rotkreuz, Switzerland) according to the protocol consisting of 2 folds for 50 seconds at 6500 rpm separated by 90 seconds of cooling. Total RNA was extracted from the frozen tissues using Nucleospin RNA II isolation kit (Macherey-Nagel, Düren, Germany), according to the manufacturer's instructions.
One microgram of total RNA was reverse-transcribed to a single strand cDNA in a final volume of 20 μL in two steps according to the manufacturer's instructions. In the first step, pre-mix with RNA, random primers, dNTPs, and water were denatured at 70şC for 5 minutes. In the second step, SuperScriptTM III Reverse Transcriptase (Cat. No. 18080-093, Invitrogen, Milan, Italy), buffer, DTT, and RNase inhibitor were added and left for 5 minutes at room temperature, followed by heating for 60 minutes at 55şC, 15 minutes at 70şC, and 5 minutes at 95şC. All reagents were obtained from Invitrogen. Negative control for reverse transcription was water instead of RNA.
Relative VEGF mRNA quantification was performed using a fluorescence-based real-time detection method at ABI Prism 7300 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Oligonucleotide primers and probe for VEGF were purchased from Applied Biosystems as Inventoried Gene Expression Assay (Assay ID Hs00900054_m1) consisting of 20 × mix of unlabeled PCR primers and TaqMan FAM probe. Oligonucleotide primers and the probe for the endogenous control β-actin gene were purchased from Applied Biosystems as TaqMan FAM/TAMRA probe as follows: β-actin F 5′ CGAGCGCGGCTACAGCTT 3′, β-actin R 5′ TCCTTAATGTCACGCACGATTT 3′, and β-actin probe 6Fam-ACCACCACGGCCGAGCGG-Tamra.
A 12-μL reaction mixture containing 2.5 μL of cDNA template, 6.25 μL TaqMan Universal PCR master mix (Cat. No. 4326708, Applied Biosystems), and 0.62 μL primer probe mixture was amplified using the following thermal cycler parameters: incubation at 50°C for 2 minutes and denaturation at 95°C for 10 minutes, followed by 40 cycles of the amplification step (denaturation at 95°C for 15 seconds and annealing/extension at 60°C for 1 minute). All amplification reactions were performed in triplicate. As negative control, samples with PCR mix and reverse transcription PCR negative control were used. To obtain the mRNA quantification of VEGF gene, results were calculated using ΔΔCT method and SDS software 1.3.1. (Applied Biosystems). The mRNA of the MDA231 mammary carcinoma cell line was used as a calibrator and the β-actin gene was used as endogenous control. To normalize the data, ΔΔCT was calculated using the mean ΔCT values of each sample from which the mean ΔCT value of calibrator was subtracted; relative quantitation value was expressed as 2- ΔΔCT. For two-way statistical analysis, VEGF gene expression was determined as low or high using the median as the cut-off value.
Statistical analysis
Statistical analysis was performed using Statistica 6.1 software (StatSoft, Inc., Tulsa, OK, USA). The distribution of data was tested for normality using Kolmogorov-Smirnov test. Pearson χ2 test was used to assess the significance of association between categorical data such as tumor histologic type, nuclear grade, and pathologic stage. The measures of central tendency for continuous data such as tumor size, Ki67 index, VEGF mRNA levels, and VEGF and NF-κB/65 immunohistochemical staining were compared by t test or Mann-Whitney U test, depending on data distribution. Pearson correlation was used to determine the association between VEGF immunohistochemical and gene expression, as well as between NF-κB/65 and VEGF immunohistochemical staining and Ki67 index. The level of statistical significance was set at P < 0.05.
Results
Immunoreactivity of VEGF, NF-κB/65, and Ki67 in renal cell tumors
Clinicopathological characteristics of renal cell tumors are presented in Table 1. VEGF immunohistochemical expression in renal cell tumors showed a perimembranous staining pattern (mean number of tumors, 20.2 ± 19.9) or diffuse pattern (mean, 42.9 ± 28.9). In general, VEGF expression was more heterogeneous in CCRCCs with strong immunoreactivity and more often observed at tumor edges, around necrosis and hemorrhagic zone. In non-CCRCCs, VEGF expression was more homogeneous, mostly showing a diffuse pattern. The difference in VEGF expression between CCRCC and non-CCRCC types was significant (P < 0.001).
Table 1.
Patohistological, immunohistochemical, and molecular characteristics of renal cell tumors
| Characteristics | CCRCC* | non-CCRCC† | P |
|---|---|---|---|
| Tumor size (cm, mean±SD) | 7.8 ± 3.6 | 6.3 ± 3.5 | 0.299‡ |
| Pathological stage (No.): | |||
| 1 | 13 | 6 | 0.443§ |
| 2 | 9 | 3 | |
| Nuclear grade (No.): | |||
| 1 | 15 | 5 | 0.505§ |
| 2 | 7 | 4 | |
| VEGF (%, mean±SD): | |||
| perimembranous | 27.1 ± 18.9 | 3.3 ± 10 | 0.001‡ |
| diffuse | 31.1 ± 22.1 | 71.7 ± 23.2 | <0.001‡ |
| Ki67 index (%, mean±SD) | 13.4 ± 9.2 | 7.9 ± 5.6 | 0.106‡ |
| NF-κB/p65 (histo-score, mean±SD): | |||
| nuclear | 103.2 ± 64.9 | 98.4 ± 63.9 | 0.852‡ |
| cytoplasmic | 214.5 ± 58.5 | 225.6 ± 58.1 | 0.637‡ |
| VEGF mRNA (RQ) (median, range)║ | 10.2 (0.3-142.2) | 2.3 (0.05-14.9) | 0.004¶ |
| Number of cases | 22 | 9 |
*Abbreviations: CCRCC – clear cell renal cell carcinoma; VEGF – vascular endothelial growth factor; NF-κB – nuclear factor-κB; SD – standard deviation.
†Papillary renal cell carcinoma, collecting duct carcinoma, and chromophobe carcinoma.
‡t test.
§Pearson χ2 test.
║Relative quantity of VEGF mRNA calculated using ΔΔCT method.
¶Mann-Whitney U-test.
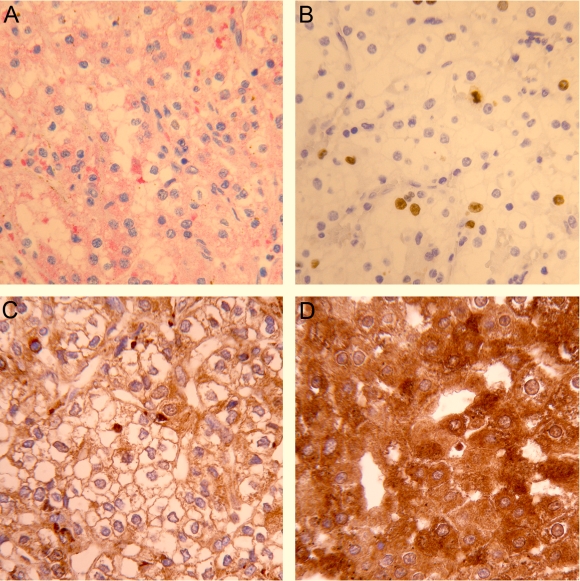
Nuclear positivity for Ki67 was variably distributed within the tumors. Double immunostaining did not show an association between Ki67 and VEGF positivity (Figure 1). Besides, there was no difference in the mean value of Ki67 proliferation index (Table 1) between CCRCCs and non-CCRCCs.
Figure 1.
Immunohistochemical expression of vascular endothelial growth factor (VEGF), Ki67, and nuclear factor kappa B (NF-κB) in renal cell carcinoma. Double immunostaining for VEGF and Ki67 shows (A) the area with cytoplasmic VEGF positive (red staining) and Ki67 nuclear negative tumor cells and (B) focus with many Ki67 positive (brown staining) and VEGF negative tumor cells. (C) NF-κB/p65 immunostaining in the renal cell carcinoma with nuclear and/or cytoplasmic staining, which is less (D) or more extensively expressed. Magnification ×400.
NF-κB/p65 was detected in the cytoplasm of normal and tumor cells. Nuclear positivity was observed in tumor cells (Figure 1); however, a very weak and rare nuclear positivity was also detected in renal tubular cells. Nuclear staining in tumor cells was considered to be a mark of NF-κB activation. The majority of renal cell tumors showed a high activation rate of NF-κB/p65, with heterogeneous distribution of staining intensity (mean H-score value 101.8 ± 63.6). Cytoplasmic NF-κB/p65 staining of different intensity was detected in almost all renal cell carcinoma cells (mean H-score value 217.7 ± 57.6). There was no significant difference between the two groups of renal cell tumors according to nuclear or cytoplasmic staining of NF-κB/p65 (Table 1).
Association between gene and protein VEGF expression in renal cell tumors
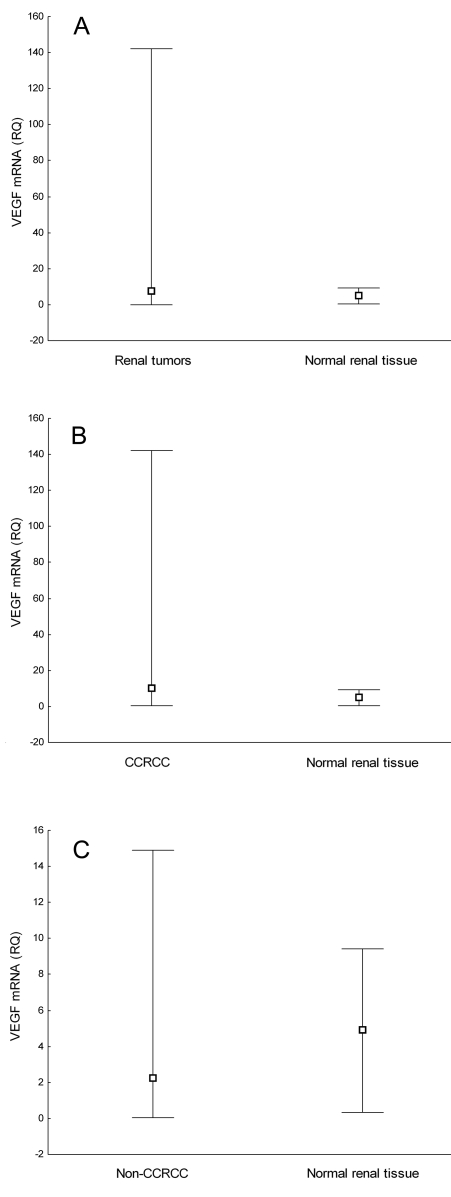
VEGF mRNA levels differed between CCRCCs and non-CCRCCs (Table 1). VEGF gene expression was significantly higher in CCRCCs (median, 10.2; range, 0.3-142.2) than in non-CCRCCs (median, 2.3; range, 0.05-14.9) (P = 0.004). There was no significant difference in VEGF gene expression either between all renal cell tumors (median, 7.7; range, 0.05-142.2) and normal renal tissue (median, 4.9; range, 0.4-9.4) or between non-CCRCCs and normal renal tissue (Figure 2). On the other hand, VEGF mRNA levels were significantly higher in CCRCCs than in normal renal tissue (P = 0.013). There was an association between VEGF protein and VEGF mRNA levels in renal cell tumors. VEGF gene was up-regulated in tumors showing perimembranous staining pattern and down-regulated in tumors displaying diffuse staining pattern (Table 2).
Figure 2.
Comparison of vascular endothelial growth factor (VEGF) messenger RNA (mRNA) relative quantity (A) between normal renal tissue and all renal cell tumors (P = 0.089, Mann-Whitney U-test), (B) between normal renal cell tissue and clear cell renal cell carcinoma (CCRC) (P = 0.013, Mann-Whitney U-test), and (C) between normal renal tissue and non-CCRCC (P = 0.676, Mann-Whitney U-test). Box represents median value and whisker plot represents range.
Table 2.
Association of immunohistochemical and gene expression of vascular endothelial growth factor (VEGF) in renal tumors (n = 31)
| Immunohistochemical VEGF expression (%) | VEGF mRNA (RQ)* |
P† | |
|---|---|---|---|
| low | high | ||
| Perimembranous (median, range) | 0 (0-70) | 30 (0-50) | 0.006 |
| Diffuse (median, range) | 60 (15-100) | 20 (0-60) | <0.001 |
*Relative quantity (RQ) of VEGF messenger RNA (mRNA) calculated using ΔΔCT method, median cut-off value for low and high VEGF is 7.7 (0.05-142.2).
†Mann-Whitney U test.
Association between NF-κB/p65, Ki67, VEGF, and clinicopathological parameters
The relationship between subcellular localization of NF-κB/p65, VEGF expression, proliferation rate of tumor cells, and clinicopathological characteristics of CCRCC is shown in Table 3. Nuclear staining of NF-κB/p65 did not show a significant association with VEGF gene expression. Cytoplasmic NF-κB/p65 positivity was found within tumor cells mostly showing diffuse VEGF staining pattern (P = 0.026) and a higher proliferation rate (P = 0.039) in patients with localized disease (P = 0.018).
Table 3.
Association of nuclear factor kappa-B (NK-κB)/p65 with pathological parameters and vascular endothelial growth factor (VEGF) in clear cell renal cell carcinoma (n = 22)
| Pathological stage* |
Ki67 index† | VEGF mRNA (RQ)*‡ |
Immunohistochemical VEGF expression† |
||||
|---|---|---|---|---|---|---|---|
| 1, 2 | 3, 4 | low | high | perimembranous | diffuse | ||
| NK-κB/p65 | 120.3 (29-247.1) | 75.2 (23.3-190.2) | 0.360 | 64.8 (23.3-152.3) | 94.2 (27-247.1) | -0.126 | 0.111 |
| P | 0.029 | 0.099 | 0.082 | 0.578 | 0.624 | ||
| Cytoplasmic NK-κB/p65 | 250 (100-300) | 200 (120-250) | 0.443 | 200 (150-300) | 200 (100-300) | -0.215 | 0.472 |
| P | 0.018 | 0.039 | 0.393 | 0.336 | 0.026 | ||
*Mann-Whitney U-test, median (range).
†rp, Pearson correlation.
‡Relative quantity of VEGF messenger RNA (mRNA) calculated using ΔΔCT method, median cut-off value for low and high is 10.2 (0.3-142.2).
Discussion
We found that immunohistochemical expression of VEGF protein is associated with relative quantity of VEGF mRNA in renal cell carcinoma. We also found a relationship between the expression of activated NF-κB and angiogenic factor in renal cell carcinoma. Since renal cell carcinoma do not respond to conventional radio- and chemotherapy, one of the many challenges in the effective management of this type of tumor is identification of new molecular markers capable of predicting tumor aggressiveness, disease progression, and therapeutic target (14). The recent developments in the understanding of the underlying biology of renal cell carcinoma have identified VEGF as a logical therapeutic target (15). On the other hand, many studies of the NF-κB pathway identified NF-κB as a therapeutic target (16,17). Our study suggests that both VEGF and NF-κB/65 should be considered as possible molecular targets in the treatment of patients with renal cell carcinoma.
We first tried to explore whether the expression of VEGF protein in tumor cells was associated with an increase in VEGF mRNA expression. Since there are different immunohistochemical phenotypes among tumor cells, it is difficult to recognize biologically different clones of tumor cells, which may be of great practical importance. Several studies investigated VEGF mRNA expression and/or expression of VEGF protein in renal cell carcinoma (14). Our results are in part consistent with previous findings, demonstrating VEGF protein in nearly 30% of CCRCCs (18,19). Besides, our study offered more information on VEGF staining pattern and differences between CCRCCs and non-CCRCCs. There was a higher heterogeneity in protein and molecular VEGF expression in CCRCCs than in non-CCRCCs, which is in accordance with previous findings that CCRCCs and non-CCRCCs behave differently (20-22).
We have already reported on the association we found between diffuse cytoplasmic staining and lower degree of vascularization and between perimembranous staining and higher degree of vascularization (22). The present study showed the up-regulation of mRNA VEGF only in renal cell tumors with perimembranous VEGF expression. This finding points to the importance of recognizing different patterns of VEGF expression, which could probably be associated with different angiogenic potential of tumor cells in neovascularization.
We also found the subcellular localization of NF-κB/p65 in renal cell carcinoma in the present study. Immunolocalization of p65 was evident in the nucleus and the cytoplasm of renal cell tumors, while normal cells displayed predominantly cytoplasmic immunolocalization, with rare and very weak nuclear immunoreactivity. This observation suggests that p65 nuclear translocation may be an important event during malignant transformation, as previously suggested (23).
NF-κB positivity has been reported in many malignancies, such as prostate cancer (24), laryngeal squamous cell carcinoma (25), pancreatic cancer (26), and breast cancer (27). NF-κB was localized both in the cytoplasm and in the nuclei of many malignant tumors (28,29), although in some of these, only cytoplasmic staining was observed (colorectal carcinoma) (30). In addition, the percentage of nuclear NF-κB also varied from only 5.6% of cells in oral squamous carcinoma (31,32) to more than 30% in prostate carcinoma (24), which is similar to ours and other studies (33).
Because nuclear localization of NF-κB is believed to be equivalent to NF-κB activation, we hypothesized that NF-κB was activated in some renal tumor cells, which may have triggered the transcription of NF-κB dependent genes and regulated the expression of corresponding proteins. We further investigated whether the expression of VEGF was associated with subcellular localization of NF-κB/p65 and found that the constitutive activity of NF-κB/p65 was linked with higher expression of VEGF mRNA. This confirmed that NF-κB/p65 is involved in the VEGF expression (10). The cytoplasmic NF-κB was associated with diffuse VEGF expression. It is difficult to determine whether immunohistochemical detection of a certain protein reflects its dynamic localization. The functional significance of this protein in the cytoplasm should be further explored. Nonetheless, the hypothesis of Nakayama et al (31) that the presence of a large amount of NF-κB in the cytoplasm may easily result in the activation of NF-κB, even by faint stimulation, seems quite reasonable.
Our study further demonstrated the association between cytoplasmic NF-κB/p65 and Ki67. It is known that NF-κB is an important regulator of cell proliferation through its direct role in the cell cycle progression (34). It has been shown that NF-κB can stimulate transcription of cyclin D, a key regulator of G1 checkpoint control, mediated by direct binding of NF-κB to multiple sites in the cyclin D1 promoter (35,36). The potential mitogen activity of VEGF in renal cell tumors was not confirmed. Even more so, on double immunostaining with VEGF and Ki67 we could not confirm the co-localization of these two markers in the same cells. This finding does not absolutely exclude the possible influence of VEGF in tumor cell proliferation, but suggests that proliferative activity in renal cell tumors is controlled by some other mitogen factors in addition to VEGF.
Finally, cytoplasmic NF-κB/p65 associated with diffuse VEGF expression, found in the present study, together with previous results (22) of higher Ki67 proliferative index, higher nuclear grade, and lower microvascular density, separate this histologically worst-differentiated renal cell carcinoma. Tumors with those histological and immunohistochemical characteristics might behave more aggressively, although in our study the pathologic stage was not worse in this group, likely because of the relatively small number of cases. On the other hand, renal cell tumors with perimembranous VEGF expression and better differentiation retain the angiogenic characteristics and the tumor contains more vasculature, similar to normal renal tissue (20,21).
In conclusion, the level of nuclear NF-κB/p65 was higher in CCRCCs than in normal renal tissue and was related to higher VEGF expression, whereas cytoplasmic NF-κB/65 was associated with a higher proliferation rate. These findings suggest that, among many different mechanisms of renal cell carcinoma progression, NF-κB may be a factor that contributes to the aggressiveness of CCRCCs via increased angiogenic secretion and proliferation of tumor cells. NF-κB, in association with VEGF, may be an important target for the development of future anti-tumor therapy for renal cell carcinoma.
Acknowledgment
This work was supported by the Ministry of Science, Education, and Sports of the Republic of Croatia (grants No. 062-0620095-0078 and 062-0620095-0082), Alpe-Adria Research Grant, and partly by the Ministry of University Education and Research grant to Giuseppe Damante. We thank Mrs Tanja Kovačević and Mr Ozren Štanfel for their excellent technical support.
References
- 1.Strefford JC, Stasevich I, Lane TM, Lu YJ, Oliver T, Young BD. A combination of molecular cytogenetic analyses reveals complex genetic alterations in conventional renal cell carcinoma. Cancer Genet Cytogenet. 2005;159:1–9. doi: 10.1016/j.cancergencyto.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Mukhopadhyay D, Datta K. Multiple regulatory pathways of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in tumors. Semin Cancer Biol. 2004;14:123–30. doi: 10.1016/j.semcancer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Costa C, Soares R, Schmitt F. Angiogenesis: now and then. APMIS. 2004;112:402–12. doi: 10.1111/j.1600-0463.2004.apm11207-0802.x. [DOI] [PubMed] [Google Scholar]
- 4.Gilmore TD. The Re1/NF-kappa B/I kappa B signal transduction pathway and cancer. Cancer Treat Res. 2003;115:241–65. doi: 10.1007/0-306-48158-8_10. [DOI] [PubMed] [Google Scholar]
- 5.Vermeulen L, De Wilde G, Notebaert S, Vanden Berghe W, Haegeman G. Regulation of the transcriptional activity of the nuclear factor-kappaB p65 subunit. Biochem Pharmacol. 2002;64:963–70. doi: 10.1016/S0006-2952(02)01161-9. [DOI] [PubMed] [Google Scholar]
- 6.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18:6853–66. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 7.Chen F, Castranova V, Shi X. New insights into the role of nuclear factor-kappaB in cell growth regulation. Am J Pathol. 2001;159:387–97. doi: 10.1016/s0002-9440(10)61708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–34. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 9.Caamano J, Hunter CA. NF-kappaB family of transcription factors: central regulators of innate and adaptive immune functions. Clin Microbiol Rev. 2002;15:414–29. doi: 10.1128/CMR.15.3.414-429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmidt D, Textor B, Pein OT, Licht AH, Andrecht S, Sator-Schmitt M, et al. Critical role for NF-kappaB-induced JunB in VEGF regulation and tumor angiogenesis. EMBO J. 2007;26:710–9. doi: 10.1038/sj.emboj.7601539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Escarcega RO, Fuentes-Alexandro S, García-Carrasco M, Gatica A, Zamora A. The transcription factor nuclear factor-kappa B and cancer. Clin Oncol (R Coll Radiol) 2007;19:154–61. doi: 10.1016/j.clon.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. WHO classification of tumours. Pathology and genetics of tumours of the urinary system and male genital organs. Vol. 6. Lyon (France): IARC Press; 2004. [Google Scholar]
- 13.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6:655–63. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Rini BI, Small EJ. Biology and clinical development of vascular endothelial growth factor-targeted therapy in renal cell carcinoma. J Clin Oncol. 2005;23:1028–43. doi: 10.1200/JCO.2005.01.186. [DOI] [PubMed] [Google Scholar]
- 15.Costa LJ, Drabkin HA. Renal cell carcinoma: new developments in molecular biology and potential for targeted therapies. Oncologist. 2007;12:1404–15. doi: 10.1634/theoncologist.12-12-1404. [DOI] [PubMed] [Google Scholar]
- 16.Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–9. doi: 10.1016/S1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 18.Paradis V, Lagha NB, Zeimoura L, Blanchet P, Eschwege P, Ba N, et al. Expression of vascular endothelial growth factor in renal cell carcinomas. Virchows Arch. 2000;436:351–6. doi: 10.1007/s004280050458. [DOI] [PubMed] [Google Scholar]
- 19.Song KH, Song J, Jeong GB, Kim JM, Jung SH, Song J. Vascular endothelial growth factor – its relation to neovascularization and their significance as prognostic factors in renal cell carcinoma. Yonsei Med J. 2001;42:539–46. doi: 10.3349/ymj.2001.42.5.539. [DOI] [PubMed] [Google Scholar]
- 20.MacLennan GT, Bostwick DG. Microvessel density in renal cell carcinoma: lack of prognostic significance. Urology. 1995;46:27–30. doi: 10.1016/S0090-4295(99)80153-8. [DOI] [PubMed] [Google Scholar]
- 21.Herbst C, Kosmehl H, Stiller KJ, Berndt A, Eiselt M, Schubert J, et al. Evaluation of microvessel density by computerised image analysis in human renal cell carcinoma. Correlation to pT category, nuclear grade, proliferative activity and occurrence of metastasis. J Cancer Res Clin Oncol. 1998;124:141–7. doi: 10.1007/s004320050147. [DOI] [PubMed] [Google Scholar]
- 22.Djordjevic G, Mozetic V, Mozetic DV, Licul V, Ilijas KM, Mustac E, et al. Prognostic significance of vascular endothelial growth factor expression in clear cell renal cell carcinoma. Pathol Res Pract. 2007;203:99–106. doi: 10.1016/j.prp.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Bindhu OS, Ramadas K, Sebastian P, Pillai MR. High expression levels of nuclear factor kappa B and gelatinases in the tumorigenesis of oral squamous cell carcinoma. Head Neck. 2006;28:916–25. doi: 10.1002/hed.20437. [DOI] [PubMed] [Google Scholar]
- 24.Lessard L, Mes-Masson AM, Lamarre L, Wall L, Lattouf JB, Saad F. NF -kappa B nuclear localization and its prognostic significance in prostate cancer. BJU Int. 2003;91:417–20. doi: 10.1046/j.1464-410X.2003.04104.x. [DOI] [PubMed] [Google Scholar]
- 25.Du J, Chen GG, Vlantis AC, Xu H, Tsang RK, van Hasselt AC. The nuclear localization of NFkappaB and p53 is positively correlated with HPV16 E7 level in laryngeal squamous cell carcinoma. J Histochem Cytochem. 2003;51:533–9. doi: 10.1177/002215540305100415. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–27. [PubMed] [Google Scholar]
- 27.Helbig G, Christopherson KW, II, Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–8. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, et al. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7:4136–42. [PubMed] [Google Scholar]
- 29.Pallares J, Martínez-Guitarte JL, Dolcet X, Llobet D, Rue M, Palacios J, et al. Abnormalities in the NF-kappaB family and related proteins in endometrial carcinoma. J Pathol. 2004;204:569–77. doi: 10.1002/path.1666. [DOI] [PubMed] [Google Scholar]
- 30.Maihöfner C, Charalambous MP, Bhambra U, Lightfoot T, Geisslinger G, Gooderham NJ, et al. Expression of cyclooxygenase-2 parallels expression of interleukin-1beta, interleukin-6 and NF-kappaB in human colorectal cancer. Carcinogenesis. 2003;24:665–71. doi: 10.1093/carcin/bgg006. [DOI] [PubMed] [Google Scholar]
- 31.Nakayama H, Ikebe T, Beppu M, Shirasuna K. High expression levels of nuclear factor kappaB, IkappaB kinase alpha and Akt kinase in squamous cell carcinoma of the oral cavity. Cancer. 2001;92:3037–44. doi: 10.1002/1097-0142(20011215)92:12<3037::AID-CNCR10171>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Peng B, Chen X. Expressions of nuclear factor kappaB, inducible nitric oxide synthase, and vascular endothelial growth factor in adenoid cystic carcinoma of salivary glands: correlations with the angiogenesis and clinical outcome. Clin Cancer Res. 2005;11:7334–43. doi: 10.1158/1078-0432.CCR-05-0241. [DOI] [PubMed] [Google Scholar]
- 33.Oya M, Takayanagi A, Horiguchi A, Mizuno R, Ohtsubo M, Marumo K, et al. Increased nuclear factor-kappa B activation is related to the tumor development of renal cell carcinoma. Carcinogenesis. 2003;24:377–84. doi: 10.1093/carcin/24.3.377. [DOI] [PubMed] [Google Scholar]
- 34.Joyce D, Albanese C, Steer J, Fu M, Bouzahzah B, Pestell RG. NF-kappaB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev. 2001;12:73–90. doi: 10.1016/S1359-6101(00)00018-6. [DOI] [PubMed] [Google Scholar]
- 35.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–99. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–8. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]