Fig. 3.
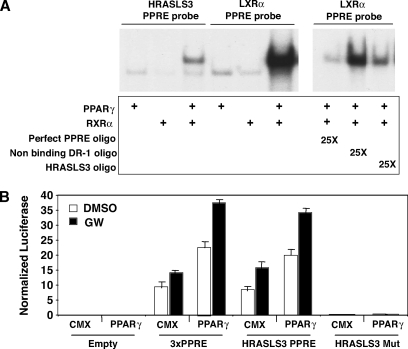
The HRASLS3 promoter contains a functional PPARγ response element. A: In vitro translated PPARγ and RXR bind to both the HRASLS3 and LXRα DR-1 sequences as a heterodimer. Twenty-five times molar excess of cold HRASLS3 DR-1 oligonucleotide effectively competes away binding of PPAR/RXR from the LXRα DR-1 in a similar manner as excess cold perfect PPRE, while a DR-1 sequence to which PPAR/RXR heterodimers do not bind is unable to displace PPAR/RXR heterodimers from the LXRα PPRE. Electrophoretic-mobility shift assays were performed using radiolabeled oligonucleotides corresponding to the direct repeat (DR-1) sequence in the promoter of HRASLS3 or LXRα, a characterized direct target of PPARγ. B: The HRASLS3 PPRE reporter construct, but not the mutant HRASL3 PPRE reporter, is activated by PPARγ in response to ligand in a manner similar to that seen for the aP2 PPRE. HEK-293T cells were transiently transfected with either empty reporter plasmid or reporter plasmids containing the aP2 PPRE, the HRASLS3 PPRE, or a mutated version of the HRASLS3 PPRE with or without a PPARγ expression plasmid. Cells were subsequently treated with ligand or vehicle for 24 h under low serum conditions and luciferase activity measured. Basal expression of the HRASLS3 reporter in the absence of transfected PPARγ is likely due to endogenous expression of PPARs. Error bars indicate ± SEM.