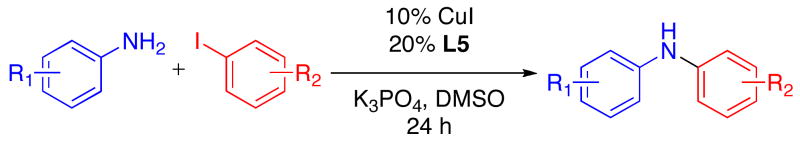
Table 2.
Cu-Catalyzed Reactions of Anilines with Aryl Iodides.a
 | ||||
|---|---|---|---|---|
| entry | product | temperature (°C) | yield (%)b | |
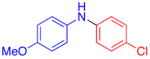
| 1 |
|
70 | 82c | |
| 2 |
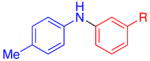
|
R=CO2Et | 80 | 78 |
| 3 | CN | 80 | 73 | |
| 4 |
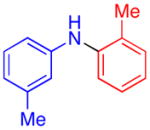
|
80 | 71 | |
| 5 |
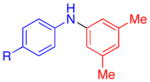
|
R=Ac | 90 | 60 |
| 6 | CO2Et | 90 | 50 | |
| 7 | CN | 90 | 52 | |
| 8 |
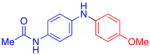
|
80 | 82 | |
| 9 |
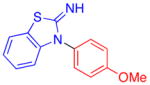
|
90 | 68 | |
Reactions Conditions: 2.0 mmol ArNH2, 1.0 mmol ArI, 2.0 mmol K3PO4, 0.10 mmol CuI, 0.20 mmol L5, 0.5 mL DMSO, in a sealed tube under an N2 atmosphere for 24 h.
Yields reported are the average of at least two runs determined to be > 95% pure by elemental analysis or 1H NMR.
5% CuI, 10% L5, 1.5 mmol ArNH2
