Table 3.
Cu-Catalyzed Reactions of Anilines with Aryl Bromides.a
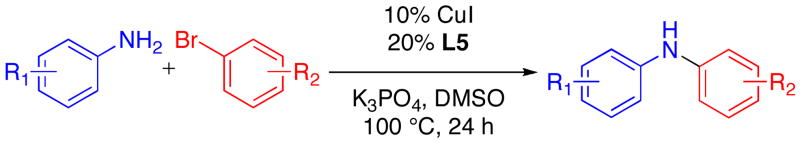 | |||
|---|---|---|---|
| entry | product | yield (%)b | |
| 1 |
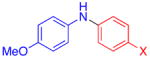
|
X = Cl | 76 |
| 2 | F | 72 | |
| 3 |
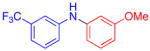
|
55 | |
| 4 |
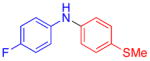
|
71 | |
| 5 |
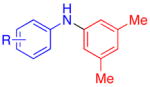
|
R = 2,5-Me2 | 75c |
| 6 | 2-OMe | 70 | |
| 7 |
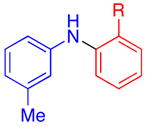
|
R = Me | 74d |
| 8 | OMe | 67d | |
| 9 |
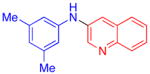
|
51 | |
Reactions Conditions: 2.0 mmol ArNH2, 1.0 mmol ArBr, 2.0 mmol K3PO4, 0.10 mmol CuI, 0.20 mmol L5, 0.5 mL DMSO, in a sealed tube under an N2 atmosphere for 24 h.
Yields reported are the average of at least two runs determined to be > 95% pure by elemental analysis or 1H NMR.
20% L15 employed as a ligand in DMF at 110 °C.
30 h.
